PARP Inhibitor Resistance Mechanisms and Implications for Post-Progression Combination Therapies
- PMID: 32722408
- PMCID: PMC7465003
- DOI: 10.3390/cancers12082054
PARP Inhibitor Resistance Mechanisms and Implications for Post-Progression Combination Therapies
Abstract
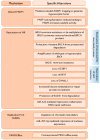
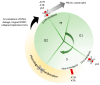
The use of PARP inhibitors (PARPi) is growing widely as FDA approvals have shifted its use from the recurrence setting to the frontline setting. In parallel, the population developing PARPi resistance is increasing. Here we review the role of PARP, DNA damage repair, and synthetic lethality. We discuss mechanisms of resistance to PARP inhibition and how this informs on novel combinations to re-sensitize cancer cells to PARPi.
Keywords: BRCA; DNA damage repair; PARP inhibitor; PARP inhibitor resistance; homologous recombination; ovarian cancer; replication fork.
Conflict of interest statement
E.K.L. declares no conflict of interest. U.A.M. reports consulting fees for Merck.
Figures
Similar articles
-
Alternate therapeutic pathways for PARP inhibitors and potential mechanisms of resistance.Exp Mol Med. 2021 Jan;53(1):42-51. doi: 10.1038/s12276-021-00557-3. Epub 2021 Jan 25. Exp Mol Med. 2021. PMID: 33487630 Free PMC article. Review.
-
Fighting resistance: post-PARP inhibitor treatment strategies in ovarian cancer.Ther Adv Med Oncol. 2023 Mar 1;15:17588359231157644. doi: 10.1177/17588359231157644. eCollection 2023. Ther Adv Med Oncol. 2023. PMID: 36872947 Free PMC article. Review.
-
PARP inhibitor resistance: the underlying mechanisms and clinical implications.Mol Cancer. 2020 Jun 20;19(1):107. doi: 10.1186/s12943-020-01227-0. Mol Cancer. 2020. PMID: 32563252 Free PMC article. Review.
-
Emerging drugs for the treatment of ovarian cancer: a focused review of PARP inhibitors.Expert Opin Emerg Drugs. 2020 Jun;25(2):165-188. doi: 10.1080/14728214.2020.1773791. Epub 2020 Jun 22. Expert Opin Emerg Drugs. 2020. PMID: 32569489 Review.
-
Use of poly ADP-ribose polymerase [PARP] inhibitors in cancer cells bearing DDR defects: the rationale for their inclusion in the clinic.J Exp Clin Cancer Res. 2016 Nov 24;35(1):179. doi: 10.1186/s13046-016-0456-2. J Exp Clin Cancer Res. 2016. PMID: 27884198 Free PMC article. Review.
Cited by
-
Subsequent management and outcomes after first-line PARP inhibitors progression in ovarian cancer patients.J Ovarian Res. 2024 Apr 1;17(1):70. doi: 10.1186/s13048-024-01400-9. J Ovarian Res. 2024. PMID: 38561819 Free PMC article.
-
PARP inhibitors in ovarian cancer: overcoming resistance with combination strategies.J Gynecol Oncol. 2022 May;33(3):e44. doi: 10.3802/jgo.2022.33.e44. Epub 2022 Mar 8. J Gynecol Oncol. 2022. PMID: 35320891 Free PMC article. Review.
-
Epigenetically Downregulated Breast Cancer Gene 2 through Acetyltransferase Lysine Acetyltransferase 2B Increases the Sensitivity of Colorectal Cancer to Olaparib.Cancers (Basel). 2023 Nov 25;15(23):5580. doi: 10.3390/cancers15235580. Cancers (Basel). 2023. PMID: 38067284 Free PMC article.
-
The USP1 Inhibitor KSQ-4279 Overcomes PARP Inhibitor Resistance in Homologous Recombination-Deficient Tumors.Cancer Res. 2024 Oct 15;84(20):3419-3434. doi: 10.1158/0008-5472.CAN-24-0293. Cancer Res. 2024. PMID: 39402989 Free PMC article.
-
SIK2 inhibition enhances PARP inhibitor activity synergistically in ovarian and triple-negative breast cancers.J Clin Invest. 2022 Jun 1;132(11):e146471. doi: 10.1172/JCI146471. J Clin Invest. 2022. PMID: 35642638 Free PMC article.
References
-
- Ghezraoui H., Oliveira C., Becker J.R., Bilham K., Moralli D., Anzilotti C., Fischer R., Deobagkar-Lele M., Sanchiz-Calvo M., Fueyo-Marcos E., et al. 53BP1 cooperation with the REV7–shieldin complex underpins DNA structure-specific NHEJ. Nature. 2018;560:122–127. doi: 10.1038/s41586-018-0362-1. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

