Mass-Spectrometry Based Proteome Comparison of Extracellular Vesicle Isolation Methods: Comparison of ME-kit, Size-Exclusion Chromatography, and High-Speed Centrifugation
- PMID: 32722497
- PMCID: PMC7459681
- DOI: 10.3390/biomedicines8080246
Mass-Spectrometry Based Proteome Comparison of Extracellular Vesicle Isolation Methods: Comparison of ME-kit, Size-Exclusion Chromatography, and High-Speed Centrifugation
Abstract
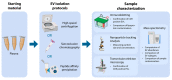
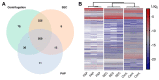
Extracellular vesicles (EVs) are small membrane-enclosed particles released by cells under various conditions specific to cells' biological states. Hence, mass-spectrometry (MS) based proteome analysis of EVs in plasma has gained much attention as a method to discover novel protein biomarkers. MS analysis of EVs in plasma is challenging and EV isolation is usually necessary. Therefore, we compared differences in abundance, subtypes, and contamination for EVs isolated by high-speed centrifugation, size exclusion chromatography (SEC), and peptide-affinity precipitation (PAP/ME kit) for subsequent MS-based proteome analysis. Successful EV isolation was evaluated by nanoparticle-tracking analysis, immunoblotting, and transmission electron microscopy, while EV abundance, EV subtypes, and contamination was evaluated by label-free tandem MS. High-speed centrifugation and SEC isolates showed high EV abundance at the expense of contamination by non-EV proteins and lipoproteins, respectively. These two methods also resulted in EVs of a similar type, however, with smaller EVs in SEC isolates. PAP isolates had a relatively low EV abundance and high contamination. We consider high-speed centrifugation and SEC suitable as EV isolation for MS biomarker studies, where the choice between the two should depend on the scientific questions and whether the focus is on larger or smaller EVs or a combination of both.
Keywords: EV isolation; Extracellular vesicles; ME kit; high-speed centrifugation; human plasma; mass spectrometry; peptide affinity; proteome; proteomics; size exclusion chromatography.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




References
-
- Kowal J., Arras G., Colombo M., Jouve M., Morath J.P., Primdal-Bengtson B., Dingli F., Loew D., Tkach M., Théry C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA. 2016;113:E968–E977. doi: 10.1073/pnas.1521230113. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

