Exosome: A New Player in Translational Nanomedicine
- PMID: 32722531
- PMCID: PMC7463834
- DOI: 10.3390/jcm9082380
Exosome: A New Player in Translational Nanomedicine
Abstract
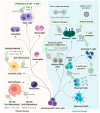
Exosomes are extracellular vesicles released by the vast majority of cell types both in vivo and ex vivo, upon the fusion of multivesicular bodies (MVBs) with the cellular plasma membrane. Two main functions have been attributed to exosomes: their capacity to transport proteins, lipids and nucleic acids between cells and organs, as well as their potential to act as natural intercellular communicators in normal biological processes and in pathologies. From a clinical perspective, the majority of applications use exosomes as biomarkers of disease. A new approach uses exosomes as biologically active carriers to provide a platform for the enhanced delivery of cargo in vivo. One of the major limitations in developing exosome-based therapies is the difficulty of producing sufficient amounts of safe and efficient exosomes. The identification of potential proteins involved in exosome biogenesis is expected to directly cause a deliberate increase in exosome production. In this review, we summarize the current state of knowledge regarding exosomes, with particular emphasis on their structural features, biosynthesis pathways, production techniques and potential clinical applications.
Keywords: CARs; cancer; exosomes; gene editing; immunotherapy; liquid biopsies.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Yáñez-Mó M., Siljander P.R.M., Andreu Z., Zavec A.B., Borràs F.E., Buzas E.I., Buzas K., Casal E., Cappello F., Carvalho J., et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

