Fecal Pollution Drives Antibiotic Resistance and Class 1 Integron Abundance in Aquatic Environments of the Bolivian Andes Impacted by Mining and Wastewater
- PMID: 32722600
- PMCID: PMC7464395
- DOI: 10.3390/microorganisms8081122
Fecal Pollution Drives Antibiotic Resistance and Class 1 Integron Abundance in Aquatic Environments of the Bolivian Andes Impacted by Mining and Wastewater
Abstract
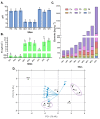
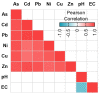
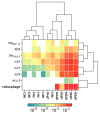
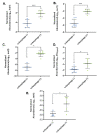
An increased abundance of antibiotic resistance genes (ARGs) in aquatic environments has been linked to environmental pollution. Mining polluted sites with high concentration of metals could favor the in situ coselection of ARGs, whereas wastewater discharges release fecal antibiotic resistant bacteria in the environment. To study the effect of human fecal contamination and mining pollution, water and sediment samples affected by mining activities and sewage discharges were collected from three lakes in Bolivia, the pristine Andean lake Pata Khota, the Milluni Chico lake directly impacted by acid mine drainage, and the Uru-Uru lake located close to Oruro city and highly polluted by mining activities and human wastewater discharges. Physicochemical parameters, including metal composition, were analyzed in water and sediment samples. ARGs were screened for and verified by quantitative polymerase chain reaction (PCR) together with the mobile element class 1 integron (intl1), as well as crAssphage, a marker of human fecal pollution. The gene intl1 was positively correlated with sul1, sul2, tetA, and blaOXA-2. CrAssphage was only detected in the Uru-Uru lake, and its tributaries and significantly higher abundance of ARGs were found in these sites. Multivariate analysis showed that crAssphage abundance, electrical conductivity, and pH were positively correlated with higher levels of intl1 and ARGs. Taken together, our results suggest that fecal pollution is the major driver of higher levels of ARGs and intl1 in environments contaminated by wastewater and mining activities.
Keywords: antibiotic resistance genes (ARGs; crAssphage; fecal pollution; metal contamination; wastewater.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources

