Renal Survival in Children with Glomerulonephritis with Crescents: A Pediatric Nephrology Research Consortium Cohort Study
- PMID: 32722612
- PMCID: PMC7464981
- DOI: 10.3390/jcm9082385
Renal Survival in Children with Glomerulonephritis with Crescents: A Pediatric Nephrology Research Consortium Cohort Study
Abstract
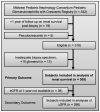
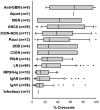
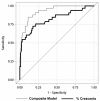
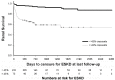
There is no evidence-based definition for diagnosing crescentic glomerulonephritis. The prognostic implications of crescentic lesions on kidney biopsy have not been quantified. Our objective was to determine risk factors for end-stage kidney disease (ESKD) in patients with glomerulonephritis and crescents on kidney biopsy. A query of the Pediatric Nephrology Research Consortium's Pediatric Glomerulonephritis with Crescents registry identified 305 patients from 15 centers. A retrospective cohort study was performed with ESKD as the primary outcome. Median age at biopsy was 11 years (range 1-21). The percentage of crescents was 3-100% (median 20%). Etiologies included IgA nephropathy (23%), lupus (21%), IgA vasculitis (19%) and ANCA-associated GN (13%), post-infectious GN (5%), and anti-glomerular basement membrane disease (3%). The prevalence of ESKD was 12% at one year and 16% at last follow-up (median = 3 years, range 1-11). Median time to ESKD was 100 days. Risk factors for ESKD included %crescents, presence of fibrous crescents, estimated GFR, and hypertension at biopsy. For each 1% increase in %crescents, there was a 3% decrease in log odds of 1-year renal survival (p = 0.003) and a 2% decrease in log odds of renal survival at last follow-up (p < 0.001). These findings provide an evidence base for enrollment criteria for crescentic glomerulonephritis in future clinical trials.
Keywords: cellular crescents; fibrous crescents; glomerulonephritis; immunosuppression; targeted biologic therapies.
Conflict of interest statement
M.N.R has received research funding from Regulus Therapeutics, Reata, Retrophin, and Novartis; W.E.S. has received funding from Pfizer; G.H. has received funding from the VMC Foundation, Amgen, Sanofi, and Alexion; S.E.W. has received funding from Bristol-Myers Squibb; all for related but separate research. None of the other authors has any relevant conflicts of interest to disclose in relation to this manuscript. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

