Discriminative Accuracy of Plasma Phospho-tau217 for Alzheimer Disease vs Other Neurodegenerative Disorders
- PMID: 32722745
- PMCID: PMC7388060
- DOI: 10.1001/jama.2020.12134
Discriminative Accuracy of Plasma Phospho-tau217 for Alzheimer Disease vs Other Neurodegenerative Disorders
Abstract
Importance: There are limitations in current diagnostic testing approaches for Alzheimer disease (AD).
Objective: To examine plasma tau phosphorylated at threonine 217 (P-tau217) as a diagnostic biomarker for AD.
Design, setting, and participants: Three cross-sectional cohorts: an Arizona-based neuropathology cohort (cohort 1), including 34 participants with AD and 47 without AD (dates of enrollment, May 2007-January 2019); the Swedish BioFINDER-2 cohort (cohort 2), including cognitively unimpaired participants (n = 301) and clinically diagnosed patients with mild cognitive impairment (MCI) (n = 178), AD dementia (n = 121), and other neurodegenerative diseases (n = 99) (April 2017-September 2019); and a Colombian autosomal-dominant AD kindred (cohort 3), including 365 PSEN1 E280A mutation carriers and 257 mutation noncarriers (December 2013-February 2017).
Exposures: Plasma P-tau217.
Main outcomes and measures: Primary outcome was the discriminative accuracy of plasma P-tau217 for AD (clinical or neuropathological diagnosis). Secondary outcome was the association with tau pathology (determined using neuropathology or positron emission tomography [PET]).
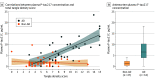
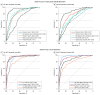
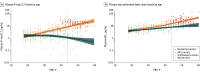
Results: Mean age was 83.5 (SD, 8.5) years in cohort 1, 69.1 (SD, 10.3) years in cohort 2, and 35.8 (SD, 10.7) years in cohort 3; 38% were women in cohort 1, 51% in cohort 2, and 57% in cohort 3. In cohort 1, antemortem plasma P-tau217 differentiated neuropathologically defined AD from non-AD (area under the curve [AUC], 0.89 [95% CI, 0.81-0.97]) with significantly higher accuracy than plasma P-tau181 and neurofilament light chain (NfL) (AUC range, 0.50-0.72; P < .05). The discriminative accuracy of plasma P-tau217 in cohort 2 for clinical AD dementia vs other neurodegenerative diseases (AUC, 0.96 [95% CI, 0.93-0.98]) was significantly higher than plasma P-tau181, plasma NfL, and MRI measures (AUC range, 0.50-0.81; P < .001) but not significantly different compared with cerebrospinal fluid (CSF) P-tau217, CSF P-tau181, and tau-PET (AUC range, 0.90-0.99; P > .15). In cohort 3, plasma P-tau217 levels were significantly greater among PSEN1 mutation carriers, compared with noncarriers, from approximately 25 years and older, which is 20 years prior to estimated onset of MCI among mutation carriers. Plasma P-tau217 levels correlated with tau tangles in participants with (Spearman ρ = 0.64; P < .001), but not without (Spearman ρ = 0.15; P = .33), β-amyloid plaques in cohort 1. In cohort 2, plasma P-tau217 discriminated abnormal vs normal tau-PET scans (AUC, 0.93 [95% CI, 0.91-0.96]) with significantly higher accuracy than plasma P-tau181, plasma NfL, CSF P-tau181, CSF Aβ42:Aβ40 ratio, and MRI measures (AUC range, 0.67-0.90; P < .05), but its performance was not significantly different compared with CSF P-tau217 (AUC, 0.96; P = .22).
Conclusions and relevance: Among 1402 participants from 3 selected cohorts, plasma P-tau217 discriminated AD from other neurodegenerative diseases, with significantly higher accuracy than established plasma- and MRI-based biomarkers, and its performance was not significantly different from key CSF- or PET-based measures. Further research is needed to optimize the assay, validate the findings in unselected and diverse populations, and determine its potential role in clinical care.
Conflict of interest statement
Figures

Comment in
-
Tau species has potential for Alzheimer disease blood test.Nat Rev Neurol. 2020 Oct;16(10):521. doi: 10.1038/s41582-020-0401-z. Nat Rev Neurol. 2020. PMID: 32792665 No abstract available.
References
-
- Global Action Plan on the Public Health Response to Dementia 2017-2025. World Health Organization; 2017.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

