Evaluation of the mRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates
- PMID: 32722908
- PMCID: PMC7449230
- DOI: 10.1056/NEJMoa2024671
Evaluation of the mRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates
Abstract
Background: Vaccines to prevent coronavirus disease 2019 (Covid-19) are urgently needed. The effect of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines on viral replication in both upper and lower airways is important to evaluate in nonhuman primates.
Methods: Nonhuman primates received 10 or 100 μg of mRNA-1273, a vaccine encoding the prefusion-stabilized spike protein of SARS-CoV-2, or no vaccine. Antibody and T-cell responses were assessed before upper- and lower-airway challenge with SARS-CoV-2. Active viral replication and viral genomes in bronchoalveolar-lavage (BAL) fluid and nasal swab specimens were assessed by polymerase chain reaction, and histopathological analysis and viral quantification were performed on lung-tissue specimens.
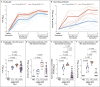
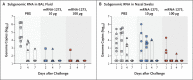
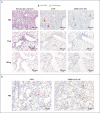
Results: The mRNA-1273 vaccine candidate induced antibody levels exceeding those in human convalescent-phase serum, with live-virus reciprocal 50% inhibitory dilution (ID50) geometric mean titers of 501 in the 10-μg dose group and 3481 in the 100-μg dose group. Vaccination induced type 1 helper T-cell (Th1)-biased CD4 T-cell responses and low or undetectable Th2 or CD8 T-cell responses. Viral replication was not detectable in BAL fluid by day 2 after challenge in seven of eight animals in both vaccinated groups. No viral replication was detectable in the nose of any of the eight animals in the 100-μg dose group by day 2 after challenge, and limited inflammation or detectable viral genome or antigen was noted in lungs of animals in either vaccine group.
Conclusions: Vaccination of nonhuman primates with mRNA-1273 induced robust SARS-CoV-2 neutralizing activity, rapid protection in the upper and lower airways, and no pathologic changes in the lung. (Funded by the National Institutes of Health and others.).
Copyright © 2020 Massachusetts Medical Society.
Figures

References
-
- Callaway E, Cyranoski D, Mallapaty S, Stoye E, Tollefson J. The coronavirus pandemic in five powerful charts. Nature 2020;579:482-483. - PubMed
-
- Mulligan MJ, Lyke KE, Kitchin N, et al. Phase 1/2 study to describe the safety and immunogenicity of a COVID-19 RNA vaccine candidate (BNT162b1) in adults 18 to 55 years of age: interim report. July 1, 2020. (https://www.medrxiv.org/content/10.1101/2020.06.30.20142570v1). preprint.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
