The H-Y Antigen in Embryonic Stem Cells Causes Rejection in Syngeneic Female Recipients
- PMID: 32723003
- PMCID: PMC7482111
- DOI: 10.1089/scd.2019.0299
The H-Y Antigen in Embryonic Stem Cells Causes Rejection in Syngeneic Female Recipients
Abstract
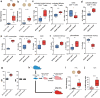
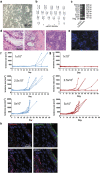
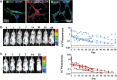
Pluripotent stem cells are promising candidates for cell-based regenerative therapies. To avoid rejection of transplanted cells, several approaches are being pursued to reduce immunogenicity of the cells or modulate the recipient's immune response. These include gene editing to reduce the antigenicity of cell products, immunosuppression of the host, or using major histocompatibility complex-matched cells from cell banks. In this context, we have investigated the antigenicity of H-Y antigens, a class of minor histocompatibility antigens encoded by the Y chromosome, to assess whether the gender of the donor affects the cell's antigenicity. In a murine transplant model, we show that the H-Y antigen in undifferentiated embryonic stem cells (ESCs), as well as ESC-derived endothelial cells, provokes T- and B cell responses in female recipients.
Keywords: Y-chromosome; allograft; immunogenicity; pluripotent stem cells; stem cell therapeutics.
Conflict of interest statement
S.S. is scientific founder and Senior Vice President of Sana Biotechnology, Inc. Neither a reagent nor any funding from Sana Biotechnology, Inc., was used in this study. The other authors declare no conflicts of interest.
Figures
Similar articles
-
UTY gene codes for an HLA-B60-restricted human male-specific minor histocompatibility antigen involved in stem cell graft rejection: characterization of the critical polymorphic amino acid residues for T-cell recognition.Blood. 2000 Nov 1;96(9):3126-32. Blood. 2000. PMID: 11049993
-
Embryonic stem cell immunogenicity increases upon differentiation after transplantation into ischemic myocardium.Circulation. 2005 Aug 30;112(9 Suppl):I166-72. doi: 10.1161/CIRCULATIONAHA.104.525824. Circulation. 2005. PMID: 16159810
-
Undifferentiated murine embryonic stem cells cannot induce portal tolerance but may possess immune privilege secondary to reduced major histocompatibility complex antigen expression.Stem Cells Dev. 2006 Oct;15(5):707-17. doi: 10.1089/scd.2006.15.707. Stem Cells Dev. 2006. PMID: 17105406
-
Clinical impact of H-Y alloimmunity.Immunol Res. 2014 May;58(2-3):249-58. doi: 10.1007/s12026-014-8514-3. Immunol Res. 2014. PMID: 24781195 Free PMC article. Review.
-
Why do some females reject males? The molecular basis for male-specific graft rejection.J Mol Med (Berl). 1997 Feb;75(2):103-14. doi: 10.1007/s001090050095. J Mol Med (Berl). 1997. PMID: 9083928 Review.
Cited by
-
Advances in immunological sorting of X and Y chromosome-bearing sperm: from proteome to sex-specific proteins.Front Vet Sci. 2025 Mar 12;12:1523491. doi: 10.3389/fvets.2025.1523491. eCollection 2025. Front Vet Sci. 2025. PMID: 40144522 Free PMC article. Review.
-
The Influence of Heterochronic Non-Myeloablative Bone Marrow Transplantation on the Immune System, Frailty, General Health, and Longevity of Aged Murine Recipients.Biomolecules. 2022 Apr 18;12(4):595. doi: 10.3390/biom12040595. Biomolecules. 2022. PMID: 35454183 Free PMC article.
-
Sex Matters-Insights from Testing Drug Efficacy in an Animal Model of Pancreatic Cancer.Cancers (Basel). 2024 May 16;16(10):1901. doi: 10.3390/cancers16101901. Cancers (Basel). 2024. PMID: 38791980 Free PMC article.
References
-
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS and Jones JM. 1998. Embryonic stem cell lines derived from human blastocysts. Science 282:1145–1147 - PubMed
-
- Takahashi K and Yamanaka S. 2006. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676 - PubMed
-
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K and Yamanaka S. 2007. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872 - PubMed
-
- Caspi O, Huber I, Kehat I, Habib M, Arbel G, Gepstein A, Yankelson L, Aronson D, Beyar R and Gepstein L. 2007. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. J Am Coll Cardiol 50:1884–1893 - PubMed
-
- Barberi T, Klivenyi P, Calingasan NY, Lee H, Kawamata H, Loonam K, Perrier AL, Bruses J, Rubio ME, et al. . 2003. Neural subtype specification of fertilization and nuclear transfer embryonic stem cells and application in parkinsonian mice. Nat Biotechnol 21:1200–1207 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

