Two distinct amphipathic peptide antibiotics with systemic efficacy
- PMID: 32723829
- PMCID: PMC7431008
- DOI: 10.1073/pnas.2005540117
Two distinct amphipathic peptide antibiotics with systemic efficacy
Abstract
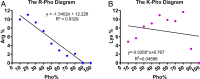
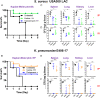
Antimicrobial peptides are important candidates for developing new classes of antibiotics because of their potency against antibiotic-resistant pathogens. Current research focuses on topical applications and it is unclear how to design peptides with systemic efficacy. To address this problem, we designed two potent peptides by combining database-guided discovery with structure-based design. When bound to membranes, these two short peptides with an identical amino acid composition can adopt two distinct amphipathic structures: A classic horizontal helix (horine) and a novel vertical spiral structure (verine). Their horizontal and vertical orientations on membranes were determined by solid-state 15N NMR data. While horine was potent primarily against gram-positive pathogens, verine showed broad-spectrum antimicrobial activity. Both peptides protected greater than 80% mice from infection-caused deaths. Moreover, horine and verine also displayed significant systemic efficacy in different murine models comparable to conventional antibiotics. In addition, they could eliminate resistant pathogens and preformed biofilms. Significantly, the peptides showed no nephrotoxicity to mice after intraperitoneal or intravenous administration for 1 wk. Our study underscores the significance of horine and verine in fighting drug-resistant pathogens.
Keywords: NMR; antibiotic resistance; nephrotoxicity; peptide antibiotics; systemic efficacy.
Conflict of interest statement
Competing interest statement: The authors have submitted worldwide patent applications for the reported new peptides.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

