CHOP and c-JUN up-regulate the mutant Z α1-antitrypsin, exacerbating its aggregation and liver proteotoxicity
- PMID: 32723872
- PMCID: PMC7504927
- DOI: 10.1074/jbc.RA120.014307
CHOP and c-JUN up-regulate the mutant Z α1-antitrypsin, exacerbating its aggregation and liver proteotoxicity
Abstract
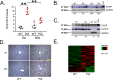
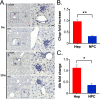
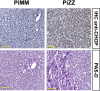
α1-Antitrypsin (AAT) encoded by the SERPINA1 gene is an acute-phase protein synthesized in the liver and secreted into the circulation. Its primary role is to protect lung tissue by inhibiting neutrophil elastase. The Z allele of SERPINA1 encodes a mutant AAT, named ATZ, that changes the protein structure and leads to its misfolding and polymerization, which cause endoplasmic reticulum (ER) stress and liver disease through a gain-of-function toxic mechanism. Hepatic retention of ATZ results in deficiency of one of the most important circulating proteinase inhibitors and predisposes to early-onset emphysema through a loss-of-function mechanism. The pathogenetic mechanisms underlying the liver disease are not completely understood. C/EBP-homologous protein (CHOP), a transcription factor induced by ER stress, was found among the most up-regulated genes in livers of PiZ mice that express ATZ and in human livers of patients homozygous for the Z allele. Compared with controls, juvenile PiZ/Chop-/- mice showed reduced hepatic ATZ and a transcriptional response indicative of decreased ER stress by RNA-Seq analysis. Livers of PiZ/Chop-/- mice also showed reduced SERPINA1 mRNA levels. By chromatin immunoprecipitations and luciferase reporter-based transfection assays, CHOP was found to up-regulate SERPINA1 cooperating with c-JUN, which was previously shown to up-regulate SERPINA1, thus aggravating hepatic accumulation of ATZ. Increased CHOP levels were detected in diseased livers of children homozygous for the Z allele. In summary, CHOP and c-JUN up-regulate SERPINA1 transcription and play an important role in hepatic disease by increasing the burden of proteotoxic ATZ, particularly in the pediatric population.
Keywords: CHOP; SERPINA1; alpha1-antitrypsin; alpha1-antitrypsin deficiency; c-JUN; c-Jun transcription factor; liver; liver injury; serpin; transcription regulation.
© 2020 Attanasio et al.
Conflict of interest statement
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

