Properties enhancement of carboxymethyl cellulose with thermo-responsive polymer as solid polymer electrolyte for zinc ion battery
- PMID: 32724055
- PMCID: PMC7387535
- DOI: 10.1038/s41598-020-69521-x
Properties enhancement of carboxymethyl cellulose with thermo-responsive polymer as solid polymer electrolyte for zinc ion battery
Abstract
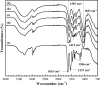
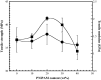
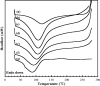
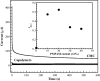
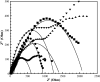
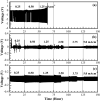
A novel polymer host from carboxymethyl cellulose (CMC)/poly(N-isopropylacrylamide) (PNiPAM) was developed for a high safety solid polymer electrolyte (SPE) in a zinc ion battery. Effects of the PNiPAM loading level in the range of 0-40% by weight ( wt%) on the chemical, mechanical, thermal, and morphological properties of the CMC/PNiPAMx films (where x is the wt% of PNiPAM) were symmetrically investigated. The obtained CMC/PNiPAMx films showed a high compatibility between the polymers. The CMC/PNiPAM20 blend showed the greatest tensile strength and modulus at 37.9 MPa and 2.1 GPa, respectively. Moreover, the thermal degradation of CMC was retarded by the addition of PNiPAM. Scanning electron microscopy images of CMC/PNiPAM20 revealed a porous structure that likely supported Zn2+ movement in the SPEs containing zinc triflate, resulting in the high Zn2+ ion transference number (0.56) and ionic conductivity (1.68 × 10-4 S cm-1). Interestingly, the presence of PNiPAM in the CMC/PNiPAMx blends showed a greater stability during charge-discharge cyclic tests, indicating the ability of PNiPAM to suppress dendrite formation from causing a short circuit. The developed CMC/PNiPAM20 based SPE is a promising material for high ionic conductivity and stability in a Zn ion battery.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Li H, Ma L, Han C, Wang Z, Liu Z, Tang Z, Zhi C. Advanced rechargeable zinc-based batteries: Recent progress and future perspectives. Nano Energy. 2019;62:550–587.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

