Protective action of Bacillus clausii probiotic strains in an in vitro model of Rotavirus infection
- PMID: 32724066
- PMCID: PMC7387476
- DOI: 10.1038/s41598-020-69533-7
Protective action of Bacillus clausii probiotic strains in an in vitro model of Rotavirus infection
Abstract
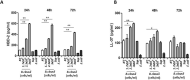
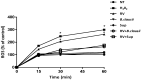
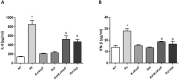
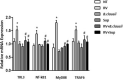
Rotavirus is the most common cause of acute gastroenteritis (AGE) in young children. Bacillus clausii (B. clausii) is a spore-forming probiotic that is able to colonize the gut. A mixture of four B. clausii strains (O/C, T, SIN and N/R) is commonly used for the treatment of AGE, and it has been demonstrated that it can reduce the duration and severity of diarrhea in children with AGE. Few studies have sought to characterize the mechanisms responsible for such beneficial effects. Intestinal effects of probiotics are likely to be strain-specific. We conducted a series of in vitro experiments investigating the activities of this mixture of B. clausii strains on biomarkers of mucosal barrier integrity and immune function in a cellular model of Rotavirus infection. B. clausii protected enterocytes against Rotavirus-induced decrease in trans-epithelial electrical resistance, and up-regulated expression of mucin 5AC and tight junction proteins (occludin and zonula occludens-1), all of which are important for effective mucosal barrier function. B. clausii also inhibited reactive oxygen species production and release of pro-inflammatory cytokines (interleukin-8 and interferon-β) in Rotavirus-infected cells, and down-regulated pro-inflammatory Toll-like receptor 3 pathway gene expression. Such mechanisms likely contributed to the observed protective effects of B. clausii against reduced cell proliferation and increased apoptosis in Rotavirus-infected enterocytes.
Conflict of interest statement
The study was supported by a Sanofi Aventis research grant devoted to the CEINGE Biotecnologie Avanzate at the University of Naples Federico II, Naples Italy. However, Sanofi Aventis had no influence on the study design, the collection, analysis, and interpretation of data, or the decision to submit the manuscript for publication
Figures



Similar articles
-
Bacillus clausii UBBC-07 reduces severity of diarrhoea in children under 5 years of age: a double blind placebo controlled study.Benef Microbes. 2019 Mar 13;10(2):149-154. doi: 10.3920/BM2018.0094. Epub 2019 Jan 14. Benef Microbes. 2019. PMID: 30638396 Clinical Trial.
-
Optimization of the treatment of rotavirus infection in children by using bacillus clausii.Wiad Lek. 2019;72(7):1320-1323. Wiad Lek. 2019. PMID: 31398163
-
Secreted Compounds of the Probiotic Bacillus clausii Strain O/C Inhibit the Cytotoxic Effects Induced by Clostridium difficile and Bacillus cereus Toxins.Antimicrob Agents Chemother. 2016 May 23;60(6):3445-54. doi: 10.1128/AAC.02815-15. Print 2016 Jun. Antimicrob Agents Chemother. 2016. PMID: 27001810 Free PMC article.
-
Bacillus clausii and gut homeostasis: state of the art and future perspectives.Expert Rev Gastroenterol Hepatol. 2016 Aug;10(8):943-8. doi: 10.1080/17474124.2016.1200465. Epub 2016 Jun 22. Expert Rev Gastroenterol Hepatol. 2016. PMID: 27291780 Review.
-
Bacillus clausii for Gastrointestinal Disorders: A Narrative Literature Review.Adv Ther. 2022 Nov;39(11):4854-4874. doi: 10.1007/s12325-022-02285-0. Epub 2022 Aug 26. Adv Ther. 2022. PMID: 36018495 Free PMC article. Review.
Cited by
-
Tight Junctions as a Key for Pathogens Invasion in Intestinal Epithelial Cells.Int J Mol Sci. 2021 Mar 2;22(5):2506. doi: 10.3390/ijms22052506. Int J Mol Sci. 2021. PMID: 33801524 Free PMC article. Review.
-
Immunomodulatory and Antioxidant Properties of a Novel Potential Probiotic Bacillus clausii CSI08.Microorganisms. 2023 Jan 18;11(2):240. doi: 10.3390/microorganisms11020240. Microorganisms. 2023. PMID: 36838205 Free PMC article.
-
Overview of the Importance of Biotics in Gut Barrier Integrity.Int J Mol Sci. 2022 Mar 7;23(5):2896. doi: 10.3390/ijms23052896. Int J Mol Sci. 2022. PMID: 35270039 Free PMC article. Review.
-
Probiotics as an Alternative to Antibiotics: Genomic and Physiological Characterization of Aerobic Spore Formers from the Human Intestine.Microorganisms. 2023 Jul 31;11(8):1978. doi: 10.3390/microorganisms11081978. Microorganisms. 2023. PMID: 37630538 Free PMC article.
-
Revisiting the Intestinal Microbiome and Its Role in Diarrhea and Constipation.Microorganisms. 2023 Aug 29;11(9):2177. doi: 10.3390/microorganisms11092177. Microorganisms. 2023. PMID: 37764021 Free PMC article. Review.
References
-
- Hartman S, Brown E, Loomis E, Russell HA. Gastroenteritis in children. Am. Fam. Physician. 2019;99:159–165. - PubMed
-
- G. B. D. Diarrhoeal Disease Collaborators Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018;18:1211–1228. doi: 10.1016/S1473-3099(18)30362-1. - DOI - PMC - PubMed
-
- Chiejina, M. & Samant, H. Viral Diarrhea. In StatPearls (2020). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

