EGCG impedes human Tau aggregation and interacts with Tau
- PMID: 32724104
- PMCID: PMC7387440
- DOI: 10.1038/s41598-020-69429-6
EGCG impedes human Tau aggregation and interacts with Tau
Abstract
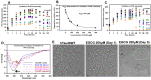
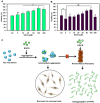
Tau aggregation and accumulation is a key event in the pathogenesis of Alzheimer's disease. Inhibition of Tau aggregation is therefore a potential therapeutic strategy to ameliorate the disease. Phytochemicals are being highlighted as potential aggregation inhibitors. Epigallocatechin-3-gallate (EGCG) is an active phytochemical of green tea that has shown its potency against various diseases including aggregation inhibition of repeat Tau. The potency of EGCG in altering the PHF assembly of full-length human Tau has not been fully explored. By various biophysical and biochemical analyses like ThS fluorescence assay, MALDI-TOF analysis and Isothermal Titration Calorimetry, we demonstrate dual effect of EGCG on aggregation inhibition and disassembly of full-length Tau and their binding affinity. The IC50 for Tau aggregation by EGCG was found to be 64.2 μM.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Lansbury PT, Lashuel HA. A century-old debate on protein aggregation and neurodegeneration enters the clinic. Nature. 2006;443:774. - PubMed
-
- Selkoe DJ. Folding proteins in fatal ways. Nature. 2003;426:900. - PubMed
-
- Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 2006;75:333–366. - PubMed
-
- Goedert M. Tau protein and the neurofibrillary pathology of Alzheimer's disease. Ann. N. Y. Acad. Sci. 1996;777:121–131. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

