PD-L1 expression in bone marrow plasma cells as a biomarker to predict multiple myeloma prognosis: developing a nomogram-based prognostic model
- PMID: 32724129
- PMCID: PMC7387472
- DOI: 10.1038/s41598-020-69616-5
PD-L1 expression in bone marrow plasma cells as a biomarker to predict multiple myeloma prognosis: developing a nomogram-based prognostic model
Abstract
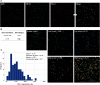
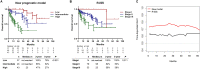
PD-L1 expression is associated with poor prognosis, although this relationship is unclear in bone marrow-derived haematologic malignancies, including multiple myeloma. We aimed to determine whether PD-L1 expression could predict the prognosis of newly diagnosed multiple myeloma (NDMM). We evaluated 126 NDMM patients (83, retrospectively; 43, prospectively) who underwent bone marrow examinations. Bone marrow aspirates were analysed for PD-L1 expression, categorized as low or high expression, using quantitative immunofluorescence. High PD-L1 expression could independently predict poor overall survival (OS) (95% CI = 1.692-8.346) in multivariate analysis. On subgroup analysis, high PD-L1 expression was associated with poor OS (95% CI = 2.283-8.761) and progression-free survival (95% CI = 1.024-3.484) in patients who did not undergo autologous stem cell transplantation (ASCT) compared with those who did. High PD-L1 expression was associated with poor OS despite frontline treatments with or without immunomodulators. Thus, PD-L1 expression can be a useful prognosis predictor in NDMM patients, whereas ASCT may be used in patients with high PD-L1 expression. We developed a prognostic nomogram and found that a combination of PD-L1 expression in bone marrow plasma cells and clinical parameters (age, cytogenetics, and lactate dehydrogenase) effectively predicted NDMM prognosis. We believe that our nomogram can help identify high-risk patients and select appropriate treatments.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Khunger M, et al. Programmed cell death 1 (PD-1) ligand (PD-L1) expression in solid tumors as a predictive biomarker of benefit from PD-1/PD-L1 axis inhibitors: a systematic review and meta-analysis. JCO Precis. Oncol. 2017;1:1–15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

