Endoplasmic Reticulum stress-dependent expression of ERO1L promotes aerobic glycolysis in Pancreatic Cancer
- PMID: 32724477
- PMCID: PMC7381747
- DOI: 10.7150/thno.45124
Endoplasmic Reticulum stress-dependent expression of ERO1L promotes aerobic glycolysis in Pancreatic Cancer
Abstract
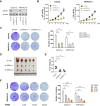
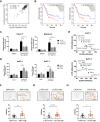
Rationale: Endoplasmic reticulum oxidoreductase 1 alpha (ERO1L) is an endoplasmic reticulum (ER) luminal glycoprotein that has a role in the formation of disulfide bonds of secreted proteins and membrane proteins. Emerging data identify ERO1L as a tumor promoter in a wide spectrum of human malignancies. However, its molecular basis of oncogenic activities remains largely unknown. Methods: Pan-cancer analysis was performed to determine the expression profile and prognostic value of ERO1L in human cancers. The mechanism by which ERO1L promotes tumor growth and glycolysis in pancreatic ductal adenocarcinoma (PDAC) was investigated by cell biological, molecular, and biochemical approaches. Results: ERO1L was highly expressed in PDAC and its precursor pancreatic intraepithelial neoplasia and acts as an independent prognostic factor for patient survival. Hypoxia and ER stress contributed to the overexpression pattern of ERO1L in PDAC. ERO1L knockdown or pharmacological inhibition with EN460 suppressed PDAC cell proliferation in vitro and slowed tumor growth in vivo. Ectopic expression of wild type ERO1L but not its inactive mutant form EROL-C394A promoted tumor growth. Bioinformatics analyses and functional analyses confirmed a regulatory role of ERO1L on the Warburg effect. Notably, inhibition of tumor glycolysis partially abrogated the growth-promoting activity of ERO1L. Mechanistically, ERO1L-mediated ROS generation was essential for its oncogenic activities. In clinical samples, ERO1L expression was correlated with the maximum standard uptake value (SUVmax) in PDAC patients who received 18F-FDG PET/CT imaging preoperatively. Analysis of TCGA cohort revealed a specific glycolysis gene expression signature that is highly correlated with unfolded protein response-related gene signature. Conclusion: Our findings uncover a key function for ERO1L in Warburg metabolism and indicate that targeting this pathway may offer alternative therapeutic strategies for PDAC.
Keywords: Aerobic glycolysis; ERO1A; ERO1α; HIF1α; Pancreatic cancer.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. - PubMed
-
- Khalaf N, Wolpin BM. Metabolic Alterations as a Signpost to Early Pancreatic Cancer. Gastroenterology. 2019;156:1560–3. - PubMed
-
- Hetz C, Papa FR. The Unfolded Protein Response and Cell Fate Control. Mol Cell. 2018;69:169–81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

