Late Pathomorphological Features of the Endocrine Pancreas in Patients With Type 2 Diabetes Mellitus
- PMID: 32724729
- PMCID: PMC7381852
- DOI: 10.7759/cureus.8777
Late Pathomorphological Features of the Endocrine Pancreas in Patients With Type 2 Diabetes Mellitus
Abstract
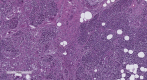
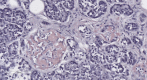
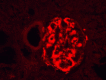
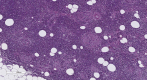
Introduction Islet amyloid polypeptide (IAPP) amyloidosis is a pathologic alteration of the pancreas, represented by abnormal accumulation of amylin in the interstitial tissue. Amylin is a neuroendocrine hormone, co-secreted with insulin by beta cells and participating in downstream regulation of postprandial glycemia. This report aims to examine IAPP amyloidosis as a late consequence of poor control of blood glucose levels in patients with type 2 diabetes mellitus (T2DM) who have been referred for autopsy. Materials and methods A total of 34 consecutive autopsies performed at the St. Marina University Hospital, Varna, Bulgaria, carried out by a single pathologist were included in the study. Samples from the tail of the pancreas were obtained to evaluate the state of the changes and were analyzed together with the specific organ changes associated with T2DM, as well as the medical documentation of the patients. Results Of the 34 autopsies, 10 cases (six females and four males) were included in the study, seven of whom had a medical history of T2D. The average age was 65.7 years (range 50 to 85 years). In all of the cases, morphological features of fibrosis and lipomatosis were present, with one of the patients having signs of pancreatic amyloidosis - Congo red positive deposition of pink, amorphous material in the extracellular matrix. Conclusion The described pathological alterations in all of the cases illustrate the progressing impairment of the structure of the pancreas, especially beta cells dysfunction in late stages of T2D, and highlight IAPP amyloidosis as the cause of irreversible damage of the isles of Langerhans and beta cell death.
Keywords: amyloid; amyloidosis; diabetes; pancreas.
Copyright © 2020, Tomova et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Basal and stimulated plasma levels of pancreatic amylin indicate its co-secretion with insulin in humans. Hartter E, Svoboda T, Ludvik B, et al. Diabetologia. 1991;34:52–54. - PubMed
-
- An islet amyloid peptide is derived from an 89-amino acid precursor by proteolytic processing. Sanke T, Bell GI, Sample C, Rubenstein AH, Steiner DF. https://www.jbc.org/content/263/33/17243.long. J Biol Chem. 1988;263:17243–17246. - PubMed
-
- Infusion of pramlintide, a human amylin analogue, delays gastric emptying in men with IDDM. Kong MF, King P, Macdonald IA, et al. Diabetologia. 1997;40:82–88. - PubMed
-
- Islet amyloid polypeptide inhibits glucagon release and exerts a dual action on insulin release from isolated islets. Åkesson B, Panagiotidis G, Westermark P, Lundquist I. Reg Pept. 2003;111:55–60. - PubMed
-
- In vitro autoradiographic localization of amylin binding sites in rat brain. Sexton PM, Paxinos G, Kenney MA, Wookey PJ, Beaumont K. Neurosci. 1994;62:553–567. - PubMed
LinkOut - more resources
Full Text Sources
