N6-methyladenosine and RNA secondary structure affect transcript stability and protein abundance during systemic salt stress in Arabidopsis
- PMID: 32724893
- PMCID: PMC7379018
- DOI: 10.1002/pld3.239
N6-methyladenosine and RNA secondary structure affect transcript stability and protein abundance during systemic salt stress in Arabidopsis
Abstract
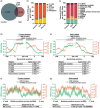
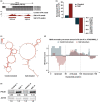
After transcription, a messenger RNA (mRNA) is further post-transcriptionally regulated by several features including RNA secondary structure and covalent RNA modifications (specifically N6-methyladenosine, m6A). Both RNA secondary structure and m6A have been demonstrated to regulate mRNA stability and translation and have been independently linked to plant responses to soil salinity levels. However, the effect of m6A on regulating RNA secondary structure and the combinatorial interplay between these two RNA features during salt stress response has yet to be studied. Here, we globally identify RNA-protein interactions and RNA secondary structure during systemic salt stress. This analysis reveals that RNA secondary structure changes significantly during salt stress, and that it is independent of global changes in RNA-protein interactions. Conversely, we find that m6A is anti-correlated with RNA secondary structure in a condition-dependent manner, with salt-specific m6A correlated with a decrease in mRNA secondary structure during salt stress. Taken together, we suggest that salt-specific m6A deposition and the associated loss of RNA secondary structure results in increases in mRNA stability for transcripts encoding abiotic stress response proteins and ultimately increases in protein levels from these stabilized transcripts. In total, our comprehensive analyses reveal important post-transcriptional regulatory mechanisms involved in plant long-term salt stress response and adaptation.
Keywords: RNA covalent modifications; RNA processing; RNA stability; RNA‐binding proteins; non‐coding RNAs; post‐transcriptional regulation.
© 2020 The Authors. Plant Direct published by American Society of Plant Biologists, Society for Experimental Biology and John Wiley & Sons Ltd.
Figures





References
-
- Anderson, S. J. , Kramer, M. C. , Gosai, S. J. , Yu, X. , Vandivier, L. E. , Nelson, A. D. L. , … Gregory, B. D. (2018). N6‐Methyladenosine inhibits local ribonucleolytic cleavage to stabilize mrnas in arabidopsis. Cell Reports, 25, 1146–1157.e3. - PubMed
-
- Chen, Q. , Zheng, Y. , Luo, L. , Yang, Y. , Hu, X. , & Kong, X. (2018). Functional FRIGIDA allele enhances drought tolerance by regulating the P5CS1 pathway in Arabidopsis thaliana. Biochemical and Biophysical Research Communications, 495, 1102–1107. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

