Blood plasma phosphorylated-tau isoforms track CNS change in Alzheimer's disease
- PMID: 32725127
- PMCID: PMC7596823
- DOI: 10.1084/jem.20200861
Blood plasma phosphorylated-tau isoforms track CNS change in Alzheimer's disease
Abstract
Highly sensitive and specific plasma biomarkers for Alzheimer's disease (AD) have the potential to improve diagnostic accuracy in the clinic and facilitate research studies including enrollment in prevention and treatment trials. We recently reported CSF tau hyperphosphorylation, especially on T217, is an accurate predictor of β-amyloidosis at asymptomatic and symptomatic stages. In the current study, we determine by mass spectrometry the potential utility of plasma p-tau isoforms to detect AD pathology and investigate CSF and plasma tau isoforms' profile relationships. Plasma tau was truncated as previously described in CSF. CSF and plasma measures of p-tau-217 and p-tau-181 were correlated. No correlation was found between CSF and plasma on total-tau levels and pS202 measures. We found p-tau-217 and p-tau-181 were highly specific for amyloid plaque pathology in the discovery cohort (n = 36, AUROC = 0.99 and 0.98 respectively). In the validation cohort (n = 92), p-tau-217 measures were still specific to amyloid status (AUROC = 0.92), and p-tau-181 measures were less specific (AUROC = 0.75).
© 2020 Barthelemy et al.
Conflict of interest statement
Disclosures: N.R. Barthélemy reported a patent to the US patent office for "blood-based assay for diagnosing and treating based on site-specific tau phosphorylation" pending, and a patent to the US patent office for "methods of diagnosing and treating based on site-specific tau phosphorylation" issued. Washington University and R.J. Bateman have equity ownership interest in C2N Diagnostics. R.J. Bateman and N.R. Barthélemy may receive royalty income based on technology (methods of diagnosing AD with phosphorylation changes) pending license by Washington University to C2N Diagnostics. R.J. Bateman receives income from C2N Diagnostics for serving on the scientific advisory board. K. Horie is a visiting scholar at Washington University and employed by Eisai Co., Ltd. K. Hori may receive income based on technology (methods of diagnosing AD with phosphorylation changes) pending license by Washington University to C2N Diagnostics. C. Sato may receive income based on technology (methods of diagnosing AD with phosphorylation changes) pending license by Washington University to C2N Diagnostics. R.J. Bateman reported "other" from C2N Diagnostics, personal fees from Eisai, AC Immune, Amgen, Pfizer, Hoffman LaRoche, and Janssen; and grants from AbbVie, Biogen, and Eli Lilly and Co. outside the submitted work. In addition, R.J. Bateman had a patent to "blood-based assay for diagnosing and treating based on site-specific tau phosphorylation" pending and a patent to "methods of diagnosing and treating based on site-specific tau phosphorylation" pending. Washington University and R.J. Bateman have equity ownership interest in C2N Diagnostics and may receive royalty income based on technology (methods of diagnosing AD with phosphorylation changes) pending license by Washington University to C2N Diagnostics.
Figures



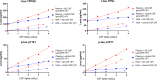
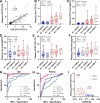
Comment in
-
Tau species has potential for Alzheimer disease blood test.Nat Rev Neurol. 2020 Oct;16(10):521. doi: 10.1038/s41582-020-0401-z. Nat Rev Neurol. 2020. PMID: 32792665 No abstract available.
References
-
- Bacioglu M., Maia L.F., Preische O., Schelle J., Apel A., Kaeser S.A., Schweighauser M., Eninger T., Lambert M., Pilotto A., et al. . 2016. Neurofilament Light Chain in Blood and CSF as Marker of Disease Progression in Mouse Models and in Neurodegenerative Diseases. Neuron. 91:56–66. 10.1016/j.neuron.2016.05.018 - DOI - PubMed
-
- Barthélemy N., Hirtz C., Schraen S., Seveno M., Bateman R., Marin P., Becher F., Gabelle A., et al. . 2015. Mass spectrometry follow-up of t181, s199, s202, t205, and T217 tau phosphorylation in cerebrospinal fluid from patients revealed a specific Alzheimer’s disease pattern. Alzheimers Dement. 11(7S_Part_19):870 10.1016/j.jalz.2015.08.063 - DOI
-
- Barthélemy N.R., Fenaille F., Hirtz C., Sergeant N., Schraen-Maschke S., Vialaret J., Buée L., Gabelle A., Junot C., Lehmann S., et al. . 2016. Tau Protein Quantification in Human Cerebrospinal Fluid by Targeted Mass Spectrometry at High Sequence Coverage Provides Insights into Its Primary Structure Heterogeneity. J. Proteome Res. 15:667–676. 10.1021/acs.jproteome.5b01001 - DOI - PubMed
-
- Barthélemy N.R., Bateman R.J., Marin P., Becher F., Sato C., Lehmann S., and Gabelle A.. 2017. Tau hyperphosphorylation on T217 in cerebrospinal fluid is specifically associated to amyloid-β pathology. bioRxiv 10.1101/226977 (Preprint posted November 30, 2017). - DOI
-
- Barthélemy N.R., Li Y., Wang G., Fagan A.M., Morris J.C., Benzinger T.L.S., Goate A., Hassenstab J., et al. . 2018. MASS SPECTROMETRY–BASED MEASUREMENT OF LONGITUDINAL CSF TAU IDENTIFIES DIFFERENT PHOSPHORYLATED SITES THAT TRACK DISTINCT STAGES OF PRESYMPTOMATIC DOMINANTLY INHERITED AD. Alzheimers Dement. 14(7S_Part_4):273-P274. 10.1016/j.jalz.2018.06.024 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

