Polysialic acid and Siglec-E orchestrate negative feedback regulation of microglia activation
- PMID: 32725371
- PMCID: PMC7904730
- DOI: 10.1007/s00018-020-03601-z
Polysialic acid and Siglec-E orchestrate negative feedback regulation of microglia activation
Abstract
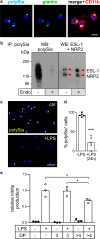
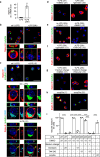
Polysialic acid (polySia) emerges as a novel regulator of microglia activity. We recently identified polysialylated proteins in the Golgi compartment of murine microglia that are released in response to inflammatory stimulation. Since exogenously added polySia is able to attenuate the inflammatory response, we proposed that the release of polysialylated proteins constitutes a mechanism for negative feedback regulation of microglia activation. Here, we demonstrate that translocation of polySia from the Golgi to the cell surface can be induced by calcium depletion of the Golgi compartment and that polysialylated proteins are continuously released for at least 24 h after the onset of inflammatory stimulation. The latter was unexpected, because polySia signals detected by immunocytochemistry are rapidly depleted. However, it indicates that the amount of released polySia is much higher than anticipated based on immunostaining. This may be crucial for microglial responses during traumatic brain injury (TBI), as we detected polySia signals in activated microglia around a stab wound in the adult mouse brain. In BV2 microglia, the putative polySia receptor Siglec-E is internalized during lipopolysaccharide (LPS)-induced activation and in response to polySia exposure, indicating interaction. Correspondingly, CRISPR/Cas9-mediated Siglec-E knockout prevents inhibition of pro inflammatory activation by exogenously added polySia and leads to a strong increase of the LPS response. A comparable increase of LPS-induced activation has been observed in microglia with abolished polySia synthesis. Together, these results indicate that the release of the microglia-intrinsic polySia pool, as implicated in TBI, inhibits the inflammatory response by acting as a trans-activating ligand of Siglec-E.
Keywords: Immune balance; Inflammatory activation; Innate immune response; Sialic acid-binding immunoglobulin-like lectins; Traumatic brain injury.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
-
- Varki A, Schnaar RL, Schauer R, et al. Sialic acids and other nonulosonic acids. In: Varki A, Cummings RD, Esko JD, et al., editors. Essentials of gycobiology. 3. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2017. pp. 179–195.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

