Interaction of HSPA5 (Grp78, BIP) with negatively charged phospholipid membranes via oligomerization involving the N-terminal end domain
- PMID: 32725381
- PMCID: PMC7385938
- DOI: 10.1007/s12192-020-01134-9
Interaction of HSPA5 (Grp78, BIP) with negatively charged phospholipid membranes via oligomerization involving the N-terminal end domain
Abstract
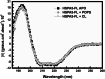
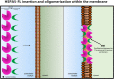
Heat shock proteins (HSPs) are ubiquitous polypeptides expressed in all living organisms that participate in several basic cellular processes, including protein folding, from which their denomination as molecular chaperones originated. There are several HSPs, including HSPA5, also known as 78-kDa glucose-regulated protein (GRP78) or binding immunoglobulin protein (BIP) that is an ER resident involved in the folding of polypeptides during their translocation into this compartment prior to the transition to the Golgi network. HSPA5 is detected on the surface of cells or secreted into the extracellular environment. Surface HSPA5 has been proposed to have various roles, such as receptor-mediated signal transduction, a co-receptor for soluble ligands, as well as a participant in tumor survival, proliferation, and resistance. Recently, surface HSPA5 has been reported to be a potential receptor of some viruses, including the novel SARS-CoV-2. In spite of these observations, the association of HSPA5 within the plasma membrane is still unclear. To gain information about this process, we studied the interaction of HSPA5 with liposomes made of different phospholipids. We found that HSPA5 has a high affinity for negatively charged phospholipids, such as palmitoyl-oleoyl phosphoserine (POPS) and cardiolipin (CL). The N-terminal and C-terminal domains of HSPA5 were independently capable of interacting with negatively charged phospholipids, but to a lesser extent than the full-length protein, suggesting that both domains are required for the maximum insertion into membranes. Interestingly, we found that the interaction of HSPA5 with negatively charged liposomes promotes an oligomerization process via intermolecular disulfide bonds in which the N-terminus end of the protein plays a critical role.
Keywords: Charged phospholipids; HSPA5; Hsp70; Liposomes; Membranes.
Figures






References
-
- Angelidis CE, Lazaridis I, Pagoulatos GN. Aggregation of hsp70 and hsc70 in vivo is distinct and temperature-dependent and their chaperone function is directly related to non-aggregated forms. Eur J Biochem. 1999;259:505–512. - PubMed
-
- Arispe N, De Maio A. ATP and ADP modulate a cation channel formed by Hsc70 I acidic phospholipid membranes. J Biol Chem. 2000;275:30839–30843. - PubMed
-
- Arispe N, Doh M, Simakova O, Kurganov B, De Maio A. Hsc70 and Hsp70 interact with phosphatidylserine on the surface of PC12 cells resulting in a decrease in viability. FASEB J. 2004;18:1636–1645. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

