Effectiveness of lanreotide autogel 120 mg at extended dosing intervals for acromegaly
- PMID: 32725444
- PMCID: PMC7674328
- DOI: 10.1007/s12020-020-02424-z
Effectiveness of lanreotide autogel 120 mg at extended dosing intervals for acromegaly
Abstract
Purpose: Recent data indicate that extended dosing intervals (EDIs) with lanreotide autogel 120 mg are effective and well-received among patients with acromegaly who have achieved biochemical control with monthly injections of long-acting somatostatin analogues (SSAs). We further evaluated the effectiveness of lanreotide autogel 120 mg delivered at EDIs (>4 weeks) in routine clinical practice.
Methods: Cross-sectional, multicentre, observational study conducted to determine the effectiveness-measured by control of serum insulin-like growth factor 1 (IGF-1)-of lanreotide autogel 120 mg at dosing intervals >4 weeks for ≥6 months in selected patients with acromegaly treated in routine clinical practice (NCT02807233). Secondary assessments included control of growth hormone (GH) levels, treatment adherence, patient satisfaction, and quality of life (QoL) using validated questionnaires (EQ-5D, AcroQoL, and TSQM-9). Patients who received radiotherapy within the last 6 months were excluded.
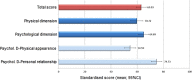
Results: Among 109 patients evaluated, mean (SD) age was 59.1 (13.2) years. IGF-1 values were normal (mean [SD]: 175.0 [74.5], 95% CI: 160.8 -189.1) in 91.7% of cases and normal in 91.4% of patients without previous radiotherapy treatment (n = 81). GH levels were ≤2.5 and ≤1 ng/mL, respectively, in 80.6% and 58.3%. Most patients were treated either every 5-6 (57.8%) or 7-8 weeks (38.5%), with 2.8% treated greater than every 8 weeks. The mean AcroQoL score was 63.0 (20.1). The mean global treatment satisfaction score (TSQM-9) was 75.1 (16.6). Treatment adherence (defined as no missed injections) was 94.5%.
Conclusion: Lanreotide autogel 120 mg at intervals of >4 weeks provided IGF-1 control in more than 90% of patients with acromegaly. Treatment satisfaction and adherence were good. These findings support use of extended dosing intervals in patients who have achieved good biochemical control with long-acting SSAs.
Keywords: Acromegaly; Growth hormone; Insulin-like growth factor 1; Lanreotide; Somatostatin.
Conflict of interest statement
I.B.: honoraria for lectures from Ipsen, Novartis and Pfizer and advisory panels from Pfizer; C.F.: honoraria for lectures from Ipsen, Novartis and Pfizer; M.M.: honoraria from lectures from Ipsen, Novartis and Pfizer; F.C.: research funding or honoraria for lectures from Ipsen, Novartis, Pfizer, Novo Nordisk, Lilly; E.M.V.: honoraria for lectures from Ipsen, Novartis and Pfizer; P.d.P.V.: honoraria for lectures or consulting fees from Pfizer and Ipsen, served on advisory panels for Ipsen; M.d.P.O.M.: honoraria for lecture from Ipsen, Novartis and Novo Nordisk; G.P.M. and I.P.d.P.: honoraria from Ipsen; D.d.C.: research grant from Pfizer, honoraria for lectures from Novartis and attended advisory board meetings for Novartis; C.R.: honoraria from Ipsen to provide clinical research services; G.D.l.C.: employee of Ipsen; C.Á.E.: honoraria as speaker fees, served on advisory boards, lecture fees and sponsorship for travel and accommodation in international scientific meetings from Ipsen, Novartis and Pfizer.
Figures


References
-
- Giustina A, Chanson P, Kleinberg D, Bronstein MD, Clemmons DR, Klibanski A, van der Lely AJ, Strasburger CJ, Lamberts SW, Ho KKY, Casanueva FF, Melmed S. Expert consensus document: A consensus on the medical treatment of acromegaly. Nat. Rev. Endocrinol. 2014;10:243–248. doi: 10.1038/nrendo.2014.21. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

