Current Understanding of the Neurobiology of Agitation
- PMID: 32726254
- PMCID: PMC7390566
- DOI: 10.5811/westjem.2020.4.45779
Current Understanding of the Neurobiology of Agitation
Abstract
Introduction: Managing agitation in the clinical setting is a challenge that many practitioners face regularly. Our evolving understanding of the etiological factors involved in aggressive acts has better informed our interventions through pharmacologic and behavioral strategies. This paper reviews the literature on the neurobiological underpinnings of aggressive behaviors, linking psychopathology with proposed mechanisms of action of psychiatric medications shown to be effective in mitigating agitation.
Methods: We performed a review of the extant literature using PubMed as a primary database. Investigation focused on neurobiology of agitation and its relation to the current evidence base for particular interventions.
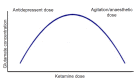
Results: There are well-established pathways that can lead to increased autonomic response and the potential for violence. Psychopathology and substance-induced perceptual distortions may lead to magnification and overestimation of environmental threat, heightening the potential for aggression. Additional challenges have arisen with the advent of several novel drugs of abuse, many of which lead to atypical clinical presentations and which can elude standard drug screens. Our interventions still lean on the evidence base found in Project BETA (Best Practices in Evaluation and Treatment of Agitation). Although not a new drug and not included in the Project BETA guidelines, ketamine and its use are also discussed, given its unique pharmacology and potential benefits when other protocoled interventions have failed.
Conclusion: Aggression can occur due to manifold reasons in the clinical setting. Having an informed understanding of the possible determinants of agitation can help with more tailored responses to individual patients, limiting the unnecessary use of medications or of interventions that could be deemed forceful.
Conflict of interest statement
Figures

References
-
- Markway EC, Baker SN. A review of the methods, interpretation, and limitations of the urine drug screen. Orthopedics. 2011;34(11):877–81. - PubMed
-
- Moeller KE, Kissack JC, Atayee RS, et al. Clinical interpretation of urine drug tests: What clinicians need to know about urine drug screens. Mayo Clin Proc. 2017;92(5):774–96. - PubMed
-
- Mathew SJ, Price RB, Charney DS. Recent advances in the neurobiology of anxiety disorders: implications for novel therapeutics. Am J Med Genet C Semin Med Genet. 2008;148c(2):89–98. - PubMed
-
- Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60(4):383–7. - PubMed
-
- Hahn A, Stein P, Windischberger C, et al. Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. Neuroimage. 2011;56(3):881–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
