Broader neutralization of CT-P27 against influenza A subtypes by combining two human monoclonal antibodies
- PMID: 32726321
- PMCID: PMC7390384
- DOI: 10.1371/journal.pone.0236172
Broader neutralization of CT-P27 against influenza A subtypes by combining two human monoclonal antibodies
Abstract
There are several broadly neutralizing monoclonal antibodies that neutralize influenza viruses with different mechanisms from traditional polyclonal antibodies induced by vaccination. CT149, which is one of the broadly neutralizing antibodies, was also previously reported to neutralize group 2 and some of group 1 influenza viruses (13 out of 13 tested group 2 viruses and 5 out of 11 group 1 viruses). In this study, we developed another antibody with the aim of compensating partial coverage of CT149 against group 1 influenza viruses. CT120 was screened among different antibody candidates and mixed with CT149. Importantly, although the binding sites of CT120 and CT149 are close to each other, the two antibodies do not interfere. The mixture of CT120 and CT149, which we named as CT-P27, showed broad efficacy by neutralizing 37 viruses from 11 different subtypes, of both group 1 and 2 influenza A viruses. Moreover, CT-P27 showed in vivo therapeutic efficacy, long prophylactic potency, and synergistic effect with oseltamivir in influenza virus-challenged mouse models. Our findings provide a novel therapeutic opportunity for more efficient treatment of influenza.
Conflict of interest statement
The funder Biotechnology Research Institute, Celltrion Inc, provided support in the form of salaries for authors [Kye Sook Yi, Pankyeom Kim, Dong-kyun Ryu, Dain Son, JiYoung Shin, HyunJoo Lee, Bok-Hyeon Im, Ji-Sang Chae, Eun Beom Lee, Soo-Young Lee]. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

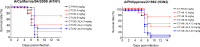
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

