A Frequency-Selective pH-Responsive paraCEST Agent
- PMID: 32726500
- PMCID: PMC7719596
- DOI: 10.1002/anie.202008888
A Frequency-Selective pH-Responsive paraCEST Agent
Abstract
Paramagnetic chemical exchange saturation transfer (paraCEST) agents are well-suited for imaging tissue pH because the basis of CEST, chemical exchange, is inherently sensitive to pH. Several previous pH-sensitive paraCEST agents were based on an exchanging Ln3+ -bound water molecule as the CEST antenna but this design often added additional line-broadening to the bulk water signal due to T2 exchange. We report herein a pH-sensitive paraCEST agent that lacks an inner-sphere water molecule but contains one Ln-bound -OH group for CEST activation. The Yb3+ complex, Yb(1), displayed a single, highly shifted CEST peak originating from the exchangeable Yb-OH proton, the frequency of which changed over the biologically relevant pH range. CEST images of phantoms ranging in pH from 6 to 8 demonstrate the potential of this agent for imaging pH. Initial rodent imaging studies showed that Gd(1) remains in the vascular system much longer than anticipated but is cleared slowly via renal filtration.
Keywords: contrast agents; imaging agents; lanthanide complexes; pH sensing; paraCEST.
© 2020 Wiley-VCH GmbH.
Conflict of interest statement
Figures


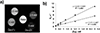



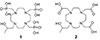
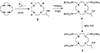
References
-
- Ward KM, Aletras AH, Balaban RS, J. Magn. Res. 2000, 143, 79–87. - PubMed
-
- Zhang S, Winter P, Wu K, Sherry AD, J. Am. Chem. Soc. 2001, 123, 1517–1518; - PubMed
- Aime S, Barge A, Botta M, De Sousa Alvaro S, Parker D, Angew. Chemie Int. Ed. 1998, 37, 2673–2675; - PubMed
- Viswanathan S, Kovacs Z, Green KN, Ratnakar SJ, Sherry AD, Chem. Rev. 2010, 110, 2960–3018; - PMC - PubMed
- Zhang S, Merritt M, Woessner DE, Lenkinski RE, Sherry AD, Acc. Chem. Res. 2003, 36, 783–790. - PubMed
-
- Wolff SD, Balaban RS, Magn. Res. Med. 1989, 10, 135–144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

