Association of COVID-19 inflammation with activation of the C5a-C5aR1 axis
- PMID: 32726800
- PMCID: PMC7116884
- DOI: 10.1038/s41586-020-2600-6
Association of COVID-19 inflammation with activation of the C5a-C5aR1 axis
Abstract
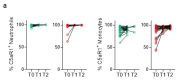
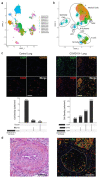
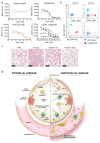
Coronavirus disease 2019 (COVID-19) is a disease caused by infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and has resulted in a pandemic1. The C5a complement factor and its receptor C5aR1 (also known as CD88) have a key role in the initiation and maintenance of several inflammatory responses by recruiting and activating neutrophils and monocytes1. Here we provide a longitudinal analysis of immune responses, including phenotypic analyses of immune cells and assessments of the soluble factors that are present in the blood and bronchoalveolar lavage fluid of patients at various stages of COVID-19 severity, including those who were paucisymptomatic or had pneumonia or acute respiratory distress syndrome. The levels of soluble C5a were increased in proportion to the severity of COVID-19 and high expression levels of C5aR1 receptors were found in blood and pulmonary myeloid cells, which supports a role for the C5a-C5aR1 axis in the pathophysiology of acute respiratory distress syndrome. Anti-C5aR1 therapeutic monoclonal antibodies prevented the C5a-mediated recruitment and activation of human myeloid cells, and inhibited acute lung injury in human C5aR1 knock-in mice. These results suggest that blockade of the C5a-C5aR1 axis could be used to limit the infiltration of myeloid cells in damaged organs and prevent the excessive lung inflammation and endothelialitis that are associated with acute respiratory distress syndrome in patients with COVID-19.
Conflict of interest statement
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

