SIRT1 Promotes Neuronal Fortification in Neurodegenerative Diseases through Attenuation of Pathological Hallmarks and Enhancement of Cellular Lifespan
- PMID: 32727328
- PMCID: PMC8686317
- DOI: 10.2174/1570159X18666200729111744
SIRT1 Promotes Neuronal Fortification in Neurodegenerative Diseases through Attenuation of Pathological Hallmarks and Enhancement of Cellular Lifespan
Abstract
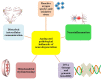
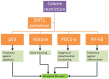
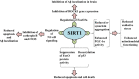
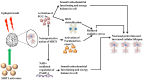
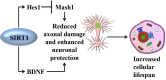
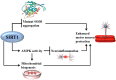
Neurodegeneration is a complex neurological phenomenon characterized by disturbed coherence in neuronal efflux. Progressive neuronal loss and brain damage due to various age-related pathological hallmarks perturb the behavioral balance and quality of life. Sirtuins have been widely investigated for their neuroprotective role, with SIRT1 being the most contemplated member of the family. SIRT1 exhibits significant capabilities to enhance neurogenesis and cellular lifespan by regulating various pathways, which makes it an exciting therapeutic target to inhibit neurodegenerative disease progression. SIRT1 mediated neuronal fortification involves modulation of molecular co-factors and biochemical pathways responsible for the induction and sustenance of pro-inflammatory and pro-oxidative environment in the cellular milieu. In this review, we present the major role played by SIRT1 in maintaining cellular strength through the regulation of genomic stability, neuronal growth, energy metabolism, oxidative stress, inhibiting mechanisms and anti-inflammatory responses. The therapeutic significance of SIRT1 has been put into perspective through a comprehensive discussion about its ameliorating potential against neurodegenerative stimuli in a variety of diseases that characteristically impair cognition, memory and motor coordination. This review enhances the acquaintance concerned with the neuroprotective potential of SIRT1 and thus promotes the development of novel SIRT1 regulating therapeutic agents and strategies.
Keywords: SIRT1; genomic stability; neuoroprotection; neurodegenerative disease.; neurogenesis; oxidative stress.
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

