The generic Informed Consent Service gICS®: implementation and benefits of a modular consent software tool to master the challenge of electronic consent management in research
- PMID: 32727514
- PMCID: PMC7391490
- DOI: 10.1186/s12967-020-02457-y
The generic Informed Consent Service gICS®: implementation and benefits of a modular consent software tool to master the challenge of electronic consent management in research
Abstract
Background: Defining and protecting participants' rights is the aim of several ethical codices and legal regulations. According to these regulations, the Informed Consent (IC) is an inevitable element of research with human subjects. In the era of "big data medicine", aspects of IC become even more relevant since research becomes more complex rendering compliance with legal and ethical regulations increasingly difficult.
Methods: Based on literature research and practical experiences gathered by the Institute for Community Medicine (ICM), University Medicine Greifswald, requirements for digital consent management systems were identified.
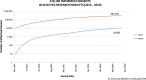
Results: To address the requirements, the free-of-charge, open-source software "generic Informed Consent Service" (gICS®) was developed by ICM to provide a tool to facilitate and enhance usage of digital ICs for the international research community covering various scenarios. gICS facilitates IC management based on IC modularisation and supports various workflows within research, including (1) electronic depiction of paper-based consents and (2) fully electronic consents. Numerous projects applied gICS and documented over 336,000 ICs and 2400 withdrawals since 2014.
Discussion: Since the consent's content is a prerequisite for securing participants' rights, application of gICS is no guarantee for legal compliance. However, gICS supports fine-granular consents and accommodation of differentiated consent states, which can be directly exchanged between systems, allowing automated data processing.
Conclusion: gICS simplifies and supports sustained IC management as a major key to successfully conduct studies and build trust in research with human subjects. Therefore, interested researchers are invited to use gICS and provide feedback for further improvements.
Keywords: Consent management; GDPR; General data protection regulation; Informed consent.
Conflict of interest statement
The authors declare that they have received funding for the development of gICS, but no competing interests exist.
Figures




References
-
- Wiesing U, Ach JS, Bormuth M, Marckmann G. Nürnberger Kodex. In: Wiesing U, editor. Ethik in der Medizin: Ein Studienbuch. Stuttgart: Reclam; 2008. pp. 123–130.
-
- Jones JH. Bad blood: The Tuskegee Syphilis Experiment. New and expanded ed. ed. New York Free Press; 1981.
-
- Reverby SM. Tuskegee’s Truths: Rethinking the Tuskegee Syphilis Study. Chapel Hill: University of North Carolina Press; 2000.
-
- National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. The Belmont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Research, Department of Health E, and Welfare; 1979 April 18, 1979. - PubMed
-
- World Medicine Association. Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects. WMA General Assembly; Fortaleza, Brazil. Fortaleza, Brazil: World Medicine Association; 2013. p. 4.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

