Efficacy of convalescent plasma for the treatment of severe influenza
- PMID: 32727526
- PMCID: PMC7388480
- DOI: 10.1186/s13054-020-03189-7
Efficacy of convalescent plasma for the treatment of severe influenza
Abstract
Background: Convalescent plasma administration may be of clinical benefit in patients with severe influenza, but reports on the efficacy of this therapy vary.
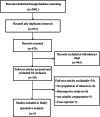
Methods: We conducted a systematic review and meta-analysis assessing randomized controlled trials (RCTs) involving the administration of convalescent plasma to treat severe influenza. Healthcare databases were searched in February 2020. All records were screened against eligibility criteria, and the risks of bias were assessed. The primary outcome was the fatality rate.
Results: A total of 2861 studies were retrieved and screened. Five eligible RCTs were identified. Pooled analyses yielded no evidence that using convalescent plasma to treat severe influenza resulted in significant reductions in mortality (odds ratio, 1.06; 95% CI, 0.51-2·23; P = 0.87; I2 = 35%), number of days in the intensive care unit, or number of days on mechanical ventilation. This treatment may have the possible benefits of increasing hemagglutination inhibition titers and reducing influenza B viral loads and cytokine levels. No serious adverse events were reported. The included studies were generally of high quality with a low risk of bias.
Conclusions: The administration of convalescent plasma appears safe but may not reduce the mortality, number of days in the intensive care unit, or number of days on mechanical ventilation in patients with severe influenza.
Keywords: Convalescent plasma; Efficacy; Meta-analysis; Severe influenza.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

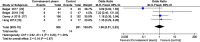
References
-
- Iuliano AD, Roguski KM, Chang HH, Muscatello DJ, Palekar R, Tempia S, Cohen C, Gran JM, Schanzer D, Cowling BJ, et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet. 2018;391(10127):1285–1300. doi: 10.1016/S0140-6736(17)33293-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- 2017ZX10204401/the National Science and Technology Major Project/International
- 81970071/National Natural Science Foundation of China/International
- 2020YFC0841300/the Special Project for Emergency of the Ministry of Science and Technology/International
- 2020B111105001/the Special Project of Guangdong Science and Technology Department/International
LinkOut - more resources
Full Text Sources
Medical

