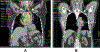
Postoperative Radiotherapy for Locally Advanced NSCLC: Implications for Shifting to Conformal, High-Risk Fields
- PMID: 32727706
- PMCID: PMC12368906
- DOI: 10.1016/j.cllc.2020.06.018
Postoperative Radiotherapy for Locally Advanced NSCLC: Implications for Shifting to Conformal, High-Risk Fields
Abstract
Background: To examine the effect of radiotherapy field size on survival outcomes and patterns of recurrence in patients treated with postoperative radiotherapy (PORT) for non-small-cell lung cancer (NSCLC).
Methods: We retrospectively reviewed the records of 216 patients with T1-4 N1-2 NSCLC following surgery and PORT using whole mediastinum (WM) or high-risk (HR) nodal fields from 1998 to 2015. Survival rates were calculated using the Kaplan-Meier method. Univariate and multivariable analyses were conducted using Cox proportional hazards modeling for outcomes and logistic regression analysis for treatment toxicities.
Results: Median follow-up was 28 months (interquartile range [IQR] 13-75 months) and 38 months (IQR 19-73 months) for WM (n = 131) and HR (n = 84) groups, respectively. Overall survival (OS) was not significantly different between groups (median OS: HR 49 vs. WM 32 months; P = .08). There was no difference in progression-free survival (PFS), freedom from locoregional recurrence (LRR), or freedom from distant metastasis (P > .2 for all). Field size was not associated with OS, PFS, or LRR (P > .40 for all). LRR rates were 20% for HR and 26% for WM groups (P = .30). There was no significant difference in patterns of initial site of LRR between groups (P > .1). WM fields (OR 3.73, P = .001) and concurrent chemotherapy (odds ratio 3.62, P = .001) were associated with grade ≥2 toxicity.
Conclusions: Locoregional control and survival rates were similar between PORT groups; an improved toxicity profile was observed in the HR group. Results from an ongoing prospective randomized clinical trial will provide further insight into the consequences of HR PORT fields.
Keywords: Locoregional control; Non–small-cell lung cancer; PORT; Patterns of recurrence; Toxicity.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of Interest
The authors report no conflicts of interest relevant to this work. S.M. reports personal fees from Oscar Health, outside the submitted work. S.H.L. reports grants from Hitachi Chemical Diagnostics and Genentech, grants and personal fees from Beyond Spring Pharmaceuticals, personal fees from AstraZeneca and Varian Medical Systems, all outside the submitted work. C.T. reports personal fees from Reflexion, AstraZeneca, and Wolter Kluwer, all outside the submitted work. In addition, C.T. holds a patent U.S. Patent (patent #9,175,079) licensed to The Board of Trustees of the Leland Stanford Junior University. J.Y.C. reports grants from BMS, personal fees from Varian Medical System and AstraZeneca, and shareholdings from Global Oncology One, all outside the submitted work. S.J.G. reports grants from Bristol Myers Squibb and Astrazeneca, and personal fees from Novocure Scientific Advisory Board, all outside the submitted work. D.R.G. reports grants and personal fees from Bristol-Myers Squibb, Varian, AstraZeneca, and Merck, personal fees from Vindico, Medscape, and Reflexion, all outside the submitted work.
Figures
References
-
- Douillard J-Y, Rosell R, De Lena M, et al. Impact of postoperative radiation therapy on survival in patients with complete resection and stage I, II, or IIIA non-small-cell lung cancer treated with adjuvant chemotherapy: the adjuvant Navelbine International Trialist Association (ANITA) Randomized Trial. Int J Radiat Oncol Biol Phys. 2008;72(3):695–701. 10.1016/j.ijrobp.2008.01.044 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials