A Thermosensitive, Phase-Variable Epigenetic Switch: pap Revisited
- PMID: 32727743
- PMCID: PMC7392537
- DOI: 10.1128/MMBR.00030-17
A Thermosensitive, Phase-Variable Epigenetic Switch: pap Revisited
Abstract
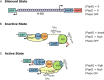
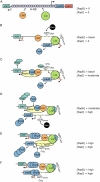
It has been more than a decade since the last comprehensive review of the phase-variable uropathogen-associated pyelonephritis-associated pilus (pap) genetic switch. Since then, important data have come to light, including additional factors that regulate pap expression, better characterization of H-NS regulation, the structure of the Lrp octamer in complex with pap regulatory DNA, the temperature-insensitive phenotype of a mutant lacking the acetyltransferase RimJ, evidence that key components of the regulatory machinery are acetylated, and new insights into the role of DNA binding by key regulators in shaping both the physical structure and regulatory state of the papI and papBA promoters. This review revisits pap, integrating these newer observations with older ones to produce a new model for the concerted behavior of this virulence-regulatory region.
Keywords: acetylation; bacteria; epigenetics; gene silencing; genetic models; histone; nucleoid-associated protein; phase variation; pili; protein acetylation; temperature; transcription.
Copyright © 2020 American Society for Microbiology.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

