SARS-CoV-2 infection in immunocompromised patients: humoral versus cell-mediated immunity
- PMID: 32727811
- PMCID: PMC7431770
- DOI: 10.1136/jitc-2020-000862
SARS-CoV-2 infection in immunocompromised patients: humoral versus cell-mediated immunity
Abstract
Background: The coronavirus disease 2019 (COVID-19) pandemic placed unprecedented pressure on various healthcare systems, including departments that use immunotherapies such as chimeric antigen receptor (CAR) T-cell therapy and immunosuppression therapy in organ transplantation units. The true impact of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection on immunocompromised CAR T-cell therapy recipients and kidney transplant recipients (KTRs) has not yet been established.
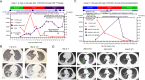
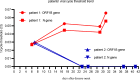
Case presentation: In this report, we compare two patients with severe COVID-19 pneumonia in either the humoral or cell-mediated immunodeficient states. The first patient was a man in his early 30s who was diagnosed with refractory multiple myeloma. He received fully humanized, anti-B-cell maturation antigen, CAR T-cell therapy before 4 months and achieved strict complete remission. He was infected with SARS-CoV-2 starting on January 26, 2019 and gradually progressed to severe pneumonia. Throughout the clinical progression of the disease, SARS-CoV-2 could not be cleared due to his humoral immunodeficient state. During this period of his severe COVID-19 pneumonia, elevated cytotoxic T-cells were observed in this patient's peripheral blood while elevated plasma levels of interleukin (IL)-2R, IL-6, tumor necrosis factor α, and ferritin were observed in his cytokine profiles. This patient eventually progressed into acute respiratory distress syndrome and recieved non-invasive ventilatory support. He failed to generate specific SARS-CoV-2 antibodies and died of respiratory failure on day 33 (d33). The second patient was a 52-year-old kidney transplant recipient (KTR) who took ciclosporin after renal transplantation for more than 7 years. He confirmed SARS-CoV-2 infection on January 20, 2019 and gradually progressed into severe pneumonia on d16 with a slightly elevated B-cell percentage and normal T-lymphocyte subsets. Viral clearance occurred together with the generation of specific anti-immunoglobulin G-SARS-CoV-2 antibodies after 2 weeks of treatment. He was symptom-free and discharged from the hospital on d42.
Conclusion: We report a CAR T-cell therapy recipient diagnosed with COVID-19 for the first time. His virus clearance failure and life-threating cytokine storm during SARS-CoV-2 infection suggested that any decision to proceed CAR T-cell therapy during COVID-19 pandemics will require extensive discussion of potential risks and benefits. Immunosuppressant treatment based on ciclosporin could be relatively safe for KTRs diagnosed with COVID-19.
Trial registration number: ChiCTR-OPN-1800018137.
Keywords: immunity, cellular; immunity, humoral; immunotherapy, adoptive; receptors, chimeric antigen.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Anti-BCMA CAR T administration in a relapsed and refractory multiple myeloma patient after COVID-19 infection: a case report.J Med Case Rep. 2021 Feb 19;15(1):90. doi: 10.1186/s13256-020-02598-0. J Med Case Rep. 2021. PMID: 33608053 Free PMC article.
-
Detection of SARS-CoV-2-Specific Humoral and Cellular Immunity in COVID-19 Convalescent Individuals.Immunity. 2020 Jun 16;52(6):971-977.e3. doi: 10.1016/j.immuni.2020.04.023. Epub 2020 May 3. Immunity. 2020. PMID: 32413330 Free PMC article.
-
Expansion of SARS-CoV-2-Specific Antibody-Secreting Cells and Generation of Neutralizing Antibodies in Hospitalized COVID-19 Patients.J Immunol. 2020 Nov 1;205(9):2437-2446. doi: 10.4049/jimmunol.2000717. Epub 2020 Sep 2. J Immunol. 2020. PMID: 32878912 Free PMC article.
-
Remodeling of the Immune Response With Aging: Immunosenescence and Its Potential Impact on COVID-19 Immune Response.Front Immunol. 2020 Aug 7;11:1748. doi: 10.3389/fimmu.2020.01748. eCollection 2020. Front Immunol. 2020. PMID: 32849623 Free PMC article. Review.
-
COVID-19, Mast Cells, Cytokine Storm, Psychological Stress, and Neuroinflammation.Neuroscientist. 2020 Oct-Dec;26(5-6):402-414. doi: 10.1177/1073858420941476. Epub 2020 Jul 18. Neuroscientist. 2020. PMID: 32684080 Review.
Cited by
-
Case Report: Severe Acute Pulmonary COVID-19 in a Teenager Post Autologous Hematopoietic Stem Cell Transplant.Front Pediatr. 2022 Mar 3;10:809061. doi: 10.3389/fped.2022.809061. eCollection 2022. Front Pediatr. 2022. PMID: 35311038 Free PMC article.
-
Immunotherapeutic approaches to curtail COVID-19.Int Immunopharmacol. 2020 Nov;88:106924. doi: 10.1016/j.intimp.2020.106924. Epub 2020 Aug 21. Int Immunopharmacol. 2020. PMID: 32877828 Free PMC article. Review.
-
Acute Respiratory Failure in Autoimmune Rheumatic Diseases: A Review.J Clin Med. 2024 May 20;13(10):3008. doi: 10.3390/jcm13103008. J Clin Med. 2024. PMID: 38792549 Free PMC article. Review.
-
Viral infection/reactivation during long-term follow-up in multiple myeloma patients with anti-BCMA CAR therapy.Blood Cancer J. 2021 Oct 18;11(10):168. doi: 10.1038/s41408-021-00563-8. Blood Cancer J. 2021. PMID: 34663791 Free PMC article. Clinical Trial. No abstract available.
-
Diagnostic Testing for SARS-CoV-2 Infection.Curr Hepatol Rep. 2021;20(4):166-174. doi: 10.1007/s11901-021-00567-9. Epub 2021 Oct 28. Curr Hepatol Rep. 2021. PMID: 34725630 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
