New insights into the evasion of host innate immunity by Mycobacterium tuberculosis
- PMID: 32728204
- PMCID: PMC7608469
- DOI: 10.1038/s41423-020-0502-z
New insights into the evasion of host innate immunity by Mycobacterium tuberculosis
Abstract
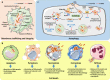
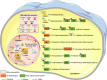
Mycobacterium tuberculosis (Mtb) is an extremely successful intracellular pathogen that causes tuberculosis (TB), which remains the leading infectious cause of human death. The early interactions between Mtb and the host innate immune system largely determine the establishment of TB infection and disease development. Upon infection, host cells detect Mtb through a set of innate immune receptors and launch a range of cellular innate immune events. However, these innate defense mechanisms are extensively modulated by Mtb to avoid host immune clearance. In this review, we describe the emerging role of cytosolic nucleic acid-sensing pathways at the host-Mtb interface and summarize recently revealed mechanisms by which Mtb circumvents host cellular innate immune strategies such as membrane trafficking and integrity, cell death and autophagy. In addition, we discuss the newly elucidated strategies by which Mtb manipulates the host molecular regulatory machinery of innate immunity, including the intranuclear regulatory machinery, the ubiquitin system, and cellular intrinsic immune components. A better understanding of innate immune evasion mechanisms adopted by Mtb will provide new insights into TB pathogenesis and contribute to the development of more effective TB vaccines and therapies.
Keywords: Autophagy; Innate immune receptors; Innate immunity; Mycobacterium tuberculosis; Ubiquitin system.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- World Health Organization. Global Tuberculosis Report 2019. (WHO, Geneva, 2019).
-
- Jasenosky LD, Scriba TJ, Hanekom WA, Goldfeld AE. T cells and adaptive immunity to Mycobacterium tuberculosis in humans. Immunol. Rev. 2015;264:74–87. - PubMed
-
- Carlos D, et al. TLR2-dependent mast cell activation contributes to the control of Mycobacterium tuberculosis infection. Microbes Infect. 2009;11:770–778. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

