A large-scale binding and functional map of human RNA-binding proteins
- PMID: 32728246
- PMCID: PMC7410833
- DOI: 10.1038/s41586-020-2077-3
A large-scale binding and functional map of human RNA-binding proteins
Erratum in
-
Author Correction: A large-scale binding and functional map of human RNA-binding proteins.Nature. 2021 Jan;589(7842):E5. doi: 10.1038/s41586-020-03067-w. Nature. 2021. PMID: 33402748 Free PMC article. No abstract available.
Abstract
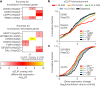
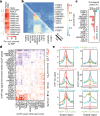
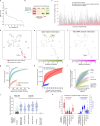
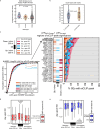
Many proteins regulate the expression of genes by binding to specific regions encoded in the genome1. Here we introduce a new data set of RNA elements in the human genome that are recognized by RNA-binding proteins (RBPs), generated as part of the Encyclopedia of DNA Elements (ENCODE) project phase III. This class of regulatory elements functions only when transcribed into RNA, as they serve as the binding sites for RBPs that control post-transcriptional processes such as splicing, cleavage and polyadenylation, and the editing, localization, stability and translation of mRNAs. We describe the mapping and characterization of RNA elements recognized by a large collection of human RBPs in K562 and HepG2 cells. Integrative analyses using five assays identify RBP binding sites on RNA and chromatin in vivo, the in vitro binding preferences of RBPs, the function of RBP binding sites and the subcellular localization of RBPs, producing 1,223 replicated data sets for 356 RBPs. We describe the spectrum of RBP binding throughout the transcriptome and the connections between these interactions and various aspects of RNA biology, including RNA stability, splicing regulation and RNA localization. These data expand the catalogue of functional elements encoded in the human genome by the addition of a large set of elements that function at the RNA level by interacting with RBPs.
Conflict of interest statement
E.L.V.N. is a co-founder, member of the Board of Directors, equity holder and paid consultant for Eclipse BioInnovations Inc. G.W.Y. is co-founder, member of the Board of Directors, on the SAB, equity holder and paid consultant for Locana and Eclipse BioInnovations Inc. G.W.Y. is a distinguished visiting professor at the National University of Singapore. E.L.V.N.’s and G.W.Y.’s interest(s) have been reviewed and approved by the University of California, San Diego in accordance with its conflict of interest policies. C.B.B. is a scientific advisory board member and equity option holder of Arrakis Therapeutics Inc. The authors declare no other competing financial interests.
Figures












References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

