Guidance on allergenicity assessment of genetically modified plants
- PMID: 32728397
- PMCID: PMC7384481
- DOI: 10.2903/j.efsa.2017.4862
Guidance on allergenicity assessment of genetically modified plants
Abstract
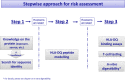
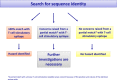
This document provides supplementary guidance on specific topics for the allergenicity risk assessment of genetically modified plants. In particular, it supplements general recommendations outlined in previous EFSA GMO Panel guidelines and Implementing Regulation (EU) No 503/2013. The topics addressed are non-IgE-mediated adverse immune reactions to foods, in vitro protein digestibility tests and endogenous allergenicity. New scientific and regulatory developments regarding these three topics are described in this document. Considerations on the practical implementation of those developments in the risk assessment of genetically modified plants are discussed and recommended, where appropriate.
Keywords: GMO; allergenicity assessment; endogenous allergenicity; guidance; newly expressed proteins.
© 2017 European Food Safety Authority. EFSA Journal published by John Wiley and Sons Ltd on behalf of European Food Safety Authority.
Figures




References
-
- Abadie V, Sollid LM, Barreiro LB and Jabri B, 2011. Integration of genetic and immunological insights into a model of celiac disease pathogenesis. Annual Review of Immunology, 29, 493–525. - PubMed
-
- van Bergen J, Mulder CJ, Mearin ML and Koning F, 2015. Local communication among mucosal immune cells in patients with celiac disease. Gastroenterol, 148, 1187–1194. - PubMed
-
- Codex Alimentarius , 2003. Foods derived from modern biotechnology. Codex Alimentarius Commission, Joint FAO/WHO Food Standards Programme, Rome.
-
- Codex Alimentarius , 2009. Foods derived from modern biotechnology. Codex Alimentarius Commission, Joint FAO/WHO Food Standards Programme, Rome.
LinkOut - more resources
Full Text Sources
