A Continuing Career in Biocatalysis: Frances H. Arnold
- PMID: 32728486
- PMCID: PMC7390471
- DOI: 10.1021/acscatal.9b02737
A Continuing Career in Biocatalysis: Frances H. Arnold
Abstract
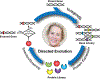
On the occasion of Professor Frances H. Arnold's recent acceptance of the 2018 Nobel Prize in Chemistry, we honor her numerous contributions to the fields of directed evolution and biocatalysis. Arnold pioneered the development of directed evolution methods for engineering enzymes as biocatalysts. Her highly interdisciplinary research has provided a ground not only for understanding the mechanisms of enzyme evolution but also for developing commercially viable enzyme biocatalysts and biocatalytic processes. In this Account, we highlight some of her notable contributions in the past three decades in the development of foundational directed evolution methods and their applications in the design and engineering of enzymes with desired functions for biocatalysis. Her work has created a paradigm shift in the broad catalysis field.
Keywords: C–H functionalization; P450s; abiological functions; biocatalysis; carbene transfer reactions; directed evolution; enzyme engineering; nitrene transfer reaction.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Bornscheuer UT; Hauer B; Jaeger KE; Schwaneberg U, Directed Evolution Empowered Redesign of Natural Proteins for the Sustainable Production of Chemicals and Pharmaceuticals. Angew Chem Int Edit 2019, 58, 36–40. - PubMed
-
- Stemmer WP, Rapid Evolution of a Protein in vitro by DNA Shuffling. Nature 1994, 370, 389–391. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
