Route of 41BB/41BBL Costimulation Determines Effector Function of B7-H3-CAR.CD28ζ T Cells
- PMID: 32728609
- PMCID: PMC7369352
- DOI: 10.1016/j.omto.2020.06.018
Route of 41BB/41BBL Costimulation Determines Effector Function of B7-H3-CAR.CD28ζ T Cells
Abstract
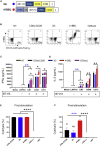
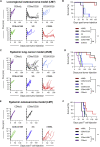
B7-H3 is actively being explored as an immunotherapy target for pediatric patients with solid tumors using monoclonal antibodies or T cells expressing chimeric antigen receptors (CARs). B7-H3-CARs containing a 41BB costimulatory domain are currently favored by several groups based on preclinical studies. In this study, we initially performed a detailed analysis of T cells expressing B7-H3-CARs with different hinge/transmembrane (CD8α versus CD28) and CD28 or 41BB costimulatory domains (CD8α/CD28, CD8α/41BB, CD28/CD28, CD28/41BB). Only subtle differences in effector function were observed between CAR T cell populations in vitro. However, CD8α/CD28-CAR T cells consistently outperformed other CAR T cell populations in three animal models, resulting in a significant survival advantage. We next explored whether adding 41BB signaling to CD8α/CD28-CAR T cells would further enhance effector function. Surprisingly, incorporating 41BB signaling into the CAR endodomain had detrimental effects, while expressing 41BBL on the surface of CD8α/CD28-CAR T cells enhanced their ability to kill tumor cells in repeat stimulation assays. Furthermore, 41BBL expression enhanced CD8α/CD28-CAR T cell expansion in vivo and improved antitumor activity in one of four evaluated models. Thus, our study highlights the intricate interplay between CAR hinge/transmembrane and costimulatory domains. Based on our study, we selected CD8α/CD28-CAR T cells expressing 41BBL for early phase clinical testing.
Keywords: B7-H3; CAR; CD276; Chimeric antigen receptor; T cell; pediatric; solid tumor.
© 2020 The Author(s).
Figures





References
-
- Ward E., DeSantis C., Robbins A., Kohler B., Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J. Clin. 2014;64:83–103. - PubMed
-
- Lagmay J.P., Krailo M.D., Dang H., Kim A., Hawkins D.S., Beaty O., 3rd, Widemann B.C., Zwerdling T., Bomgaars L., Langevin A.M. Outcome of patients with recurrent osteosarcoma enrolled in seven phase II trials through Children’s Cancer Group, Pediatric Oncology Group, and Children’s Oncology Group: learning from the past to move forward. J. Clin. Oncol. 2016;34:3031–3038. - PMC - PubMed
-
- Stahl M., Ranft A., Paulussen M., Bölling T., Vieth V., Bielack S., Görtitz I., Braun-Munzinger G., Hardes J., Jürgens H., Dirksen U. Risk of recurrence and survival after relapse in patients with Ewing sarcoma. Pediatr. Blood Cancer. 2011;57:549–553. - PubMed
-
- Pappo A.S., Anderson J.R., Crist W.M., Wharam M.D., Breitfeld P.P., Hawkins D., Raney R.B., Womer R.B., Parham D.M., Qualman S.J., Grier H.E. Survival after relapse in children and adolescents with rhabdomyosarcoma: a report from the Intergroup Rhabdomyosarcoma Study Group. J. Clin. Oncol. 1999;17:3487–3493. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

