Dimethyl-2-oxoglutarate improves redox balance and mitochondrial function in muscle pericytes of individuals with diabetes mellitus
- PMID: 32728894
- PMCID: PMC7476972
- DOI: 10.1007/s00125-020-05230-4
Dimethyl-2-oxoglutarate improves redox balance and mitochondrial function in muscle pericytes of individuals with diabetes mellitus
Abstract
Aims/hypothesis: Treatment of vascular complications of diabetes remains inadequate. We reported that muscle pericytes (MPs) from limb muscles of vascular patients with diabetes mellitus display elevated levels of oxidative stress causing a dysfunctional phenotype. Here, we investigated whether treatment with dimethyl-2-oxoglutarate (DM-2OG), a tricarboxylic acid cycle metabolite with antioxidant properties, can restore a healthy metabolic and functional phenotype.
Methods: MPs were isolated from limb muscles of diabetes patients with vascular disease (D-MPs) and from non-diabetic control participants (ND-MPs). Metabolic status was assessed in untreated and DM-2OG-treated (1 mmol/l) cells using an extracellular flux analyser and anion-exchange chromatography-mass spectrometry (IC-MS/MS). Redox status was measured using commercial kits and IC-MS/MS, with antioxidant and metabolic enzyme expression assessed by quantitative RT-PCR and western blotting. Myogenic differentiation and proliferation and pericyte-endothelial interaction were assessed as functional readouts.
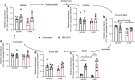
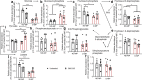
Results: D-MPs showed mitochondrial dysfunction, suppressed glycolytic activity and reduced reactive oxygen species-buffering capacity, but no suppression of antioxidant systems when compared with ND-MP controls. DM-2OG supplementation improved redox balance and mitochondrial function, without affecting glycolysis or antioxidant systems. Nonetheless, this was not enough for treated D-MPs to regain the level of proliferation and myogenic differentiation of ND-MPs. Interestingly, DM-2OG exerted a positive effect on pericyte-endothelial cell interaction in the co-culture angiogenesis assay, independent of the diabetic status.
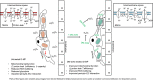
Conclusions/interpretation: These novel findings support the concept of using DM-2OG supplementation to improve pericyte redox balance and mitochondrial function, while concurrently allowing for enhanced pericyte-endothelial crosstalk. Such effects may help to prevent or slow down vasculopathy in skeletal muscles of people with diabetes. Graphical abstract.
Keywords: 2-Oxoglutarate; Diabetes mellitus; Mitochondria; Pericytes; Redox; Vascular protection.
Figures






References
-
- Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

