Oncologist perspectives on chemotherapy-induced nausea and vomiting (CINV) management and outcomes: A quantitative market research-based survey
- PMID: 32729252
- PMCID: PMC7941545
- DOI: 10.1002/cnr2.1127
Oncologist perspectives on chemotherapy-induced nausea and vomiting (CINV) management and outcomes: A quantitative market research-based survey
Abstract
Background: Chemotherapy-induced nausea and vomiting (CINV) is a distressing side effect that can negatively impact patients' quality of life and could discourage completion of chemotherapy, thereby affecting overall treatment outcomes. Although adherence to antiemetic guidelines can reduce CINV incidence in patients receiving highly or moderately emetogenic chemotherapy, CINV control remains inadequate.
Aims: The objectives of this survey were to determine oncologists' practice patterns in CINV management, identify factors that contribute to antiemetic treatment failure, and determine the outcomes of uncontrolled CINV on health care resource utilisation and on patients' attitude towards chemotherapy.
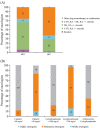
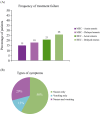
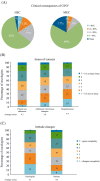
Methods and results: Quantitative market research was performed using an online questionnaire. Responses from 300 European oncologists who prescribe antiemetics and see ≥50 patients/month were analysed. Results showed that the main reasons reported by oncologists for antiemetic treatment failure were underestimating the emetogenic potential of chemotherapy, utilising weaker antiemetic regimens than required, and patient non-adherence because of administration mistakes or missed/delayed doses. Educational initiatives for the oncology multidisciplinary team may help improve guideline-consistent prescribing. Also, the availability of simpler, more convenient antiemetic therapies may improve guideline adherence and patient compliance during home administration.
Conclusion: Achieving effective CINV control is a crucial goal to improve patients' quality of life, which should optimise chemotherapy outcomes, and would ultimately reduce health care costs.
Keywords: adherence; antiemetic therapy; chemotherapy‐induced nausea and vomiting (CINV); compliance; guidelines.
© 2018 The Authors Cancer Reports Published by Wiley Periodicals, Inc.
Conflict of interest statement
M. Aapro: advisor for Eisai, Helsinn, Merck, Mundipharma, Roche, and Tesaro; honoraria from Eisai, Helsinn, Merck, Mundipharma, Roche, and Tesaro; and has received grants from Helsinn, Merck, Roche, and Tesaro.
P. Ruffo: Helsinn Healthcare SA employee.
R. Panteri: no conflicts of interest.
S. Costa: no conflicts of interest.
V. Piovesana: Helsinn Healthcare SA employee.
Figures


References
-
- Roila F, Molassiotis A, Herrstedt J, et al. Participants of the MASCC/ESMO Consensus Conference Copenhagen 2015. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy‐ and radiotherapy‐induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol. 2016;27(suppl 5):v119‐v133. 10.1093/annonc/mdw270 - DOI - PubMed
-
- National Comprehensive Cancer Network . (2018). NCCN clinical practice guidelines in oncology (NCCN guidelines®). Antiemesis. Version 2.2018. Available from: https://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf. [last accessed April 2018].
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

