Hetero-Diels-Alder Cycloaddition with RAFT Polymers as Bioconjugation Platform
- PMID: 32729643
- PMCID: PMC7693046
- DOI: 10.1002/anie.202005747
Hetero-Diels-Alder Cycloaddition with RAFT Polymers as Bioconjugation Platform
Abstract
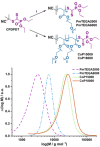
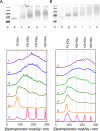
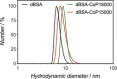
We introduce the bioconjugation of polymers synthesized by RAFT polymerization, bearing no specific functional end group, by means of hetero-Diels-Alder cycloaddition through their inherent terminal thiocarbonylthio moiety with a diene-modified model protein. Quantitative conjugation occurs over the course of a few hours, at ambient temperature and neutral pH, and in the absence of any catalyst. Our technology platform affords thermoresponsive bioconjugates, whose aggregation is solely controlled by the polymer chains.
Keywords: Diels-Alder cycloaddition; bioconjugation; end group; polymer; protein.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
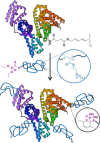
References
-
- Manning M. C., Chou D. K., Murphy B. M., Payne R. W., Katayama D. S., Pharm. Res. 2010, 27, 544–575. - PubMed
-
- Le Droumaguet B., Nicolas J., Polym. Chem. 2010, 1, 563–598.
-
- Gauthier M. A., Klok H.-A., Polym. Chem. 2010, 1, 1352–1373.
-
- Russell A. J., Baker S. L., Colina C. M., Figg C. A., Kaar J. L., Matyjaszewski K., Simakova A., Sumerlin B. S., AIChE J. 2018, 64, 3230–3245.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

