The phytochemical epigallocatechin gallate prolongs the lifespan by improving lipid metabolism, reducing inflammation and oxidative stress in high-fat diet-fed obese rats
- PMID: 32729662
- PMCID: PMC7511879
- DOI: 10.1111/acel.13199
The phytochemical epigallocatechin gallate prolongs the lifespan by improving lipid metabolism, reducing inflammation and oxidative stress in high-fat diet-fed obese rats
Abstract
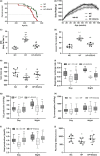
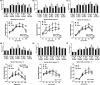
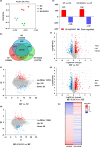
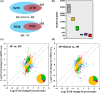
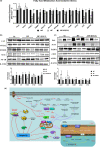
We have recently reported that epigallocatechin gallate (EGCG) could extend lifespan in healthy rats. This study aimed to investigate the effects and mechanisms of a high dose of EGCG in extending the lifespan of obese rats. Ninety adult male Wistar rats were randomly divided into the control (NC), high-fat (HF) and EGCG groups. Serum glucose and lipids, inflammation and oxidative stress were dynamically determined from adulthood to death, and the transcriptome and proteome of the liver were also examined. The median lifespans of the NC, HF and EGCG groups were 693, 599 and 683 days, respectively, and EGCG delayed death by 84 days in obese rats. EGCG improved serum glucose and lipids and reduced inflammation and oxidative stress associated with aging in obese rats induced by a high-fat diet. EGCG also significantly decreased the levels of total free fatty acids (FFAs), SFAs and the n-6/n-3 ratio but significantly increased the n-3 FFAs related to longevity. The joint study of the transcriptome and proteome in liver found that EGCG exerted its effects mainly by regulating the suppression of hydrogen peroxide and oxygen species metabolism, suppression of oxidative stress, activation of fatty acid transport and oxidation and cholesterol metabolism. EGCG significantly increased the protein expression of FOXO1, Sirt1, CAT, FABP1, GSTA2, ACSL1 and CPT2 but significantly decreased NF-κB, ACC1 and FAS protein levels in the livers of rats. All the results indicate that EGCG extends lifespan by improving FFA metabolism and reducing the levels of inflammatory and oxidative stress in obese rats.
Keywords: EGCG; free fatty acid; high-fat dietary; lifespan; proteomics; transcriptome.
© 2020 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures

References
-
- Ashida, H. , Furuyashiki, T. , Nagayasu, H. , Bessho, H. , Sakakibara, H. , Hashimoto, T. , & Kanazawa, K. (2004). Anti‐obesity actions of green tea: possible involvements in modulation of the glucose uptake system and suppression of the adipogenesis‐related transcription factors. BioFactors, 22, 135–140. 10.1002/biof.5520220126 - DOI - PubMed
-
- Bernardes de Jesus, B. , Vera, E. , Schneeberger, K. , Tejera, A. M. , Ayuso, E. , Bosch, F. , & Blasco, M. A. (2012). Telomerase gene therapy in adult and old mice delays aging and increases longevity without increasing cancer. EMBO Molecular Medicine., 4(8), 691–704. 10.1002/emmm.201200245 - DOI - PMC - PubMed
-
- Bose, M. , Lambert, J. D. , Ju, J. , Reuhl, K. R. , Shapses, S. A. , & Yang, C. S. (2008). The major green tea polyphenol, (‐)‐epigallocatechin‐3‐gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high‐fat‐fed mice. The Journal of Nutrition, 138(9), 1677–1683. 10.1093/jn/138.9.1677 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

