A novel circRNA, circNUP98, a potential biomarker, acted as an oncogene via the miR-567/ PRDX3 axis in renal cell carcinoma
- PMID: 32729669
- PMCID: PMC7520319
- DOI: 10.1111/jcmm.15629
A novel circRNA, circNUP98, a potential biomarker, acted as an oncogene via the miR-567/ PRDX3 axis in renal cell carcinoma
Abstract
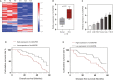
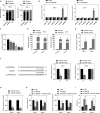
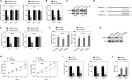
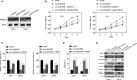
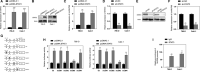
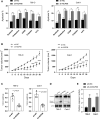
In recent years, plenty of studies found that circular RNAs (circRNAs) were essential players in the initiation and progression of various cancers including the renal cell carcinoma (RCC). However, the knowledge about the circRNAs in carcinogenesis is still limited. Dysregulated expression of circNUP98 in RCC tissues was identified by the circular RNA microarray. RT-PCR was performed to measure the expression of circNUP98 in 78 pairs of RCC tissues and adjacent normal tissues. Survival analysis was conducted to explore the association between the expression of circNUP98 and the prognosis of RCC. The function and underlying mechanisms of circSMC3 in RCC cells were investigated by RNAi, CCK-8, Western blotting, bioinformatic analysis, ChIP assay, circRIP assay and dual luciferase reporter assay. CircNUP98 was up-regulated in both RCC tissues and cell lines, and high expression of circNUP98 was correlated with poor prognosis of RCC patients. Silencing of circSMC3 inhibited the proliferation and promoted the apoptosis in a caspase-dependent manner in RCC cells. Mechanistically, we revealed that silencing of circ NUP98 inhibited RCC progression by down-regulating of PRDX3 via up-regulation of miR-567. Furthermore, STAT3 was identified as an inducer of circ NUP98 in RCC cells. CircNUP98 acts as an oncogene by a novel STAT3/circ NUP98/miR-567/PRDX3 axis, which may provide a potential biomarker and therapeutic target for the treatment of RCC.
Keywords: PRDX3; circNUP98; miR-567; renal cell carcinoma.
© 2020 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors confirm that there are no conflict of interest.
Figures

Similar articles
-
Down-Regulation of Circular RNA_000926 Attenuates Renal Cell Carcinoma Progression through miRNA-411-Dependent CDH2 Inhibition.Am J Pathol. 2019 Dec;189(12):2469-2486. doi: 10.1016/j.ajpath.2019.06.016. Epub 2019 Aug 30. Am J Pathol. 2019. PMID: 31476285
-
Circular RNA hsa_circ_0054537 sponges miR-130a-3p to promote the progression of renal cell carcinoma through regulating cMet pathway.Gene. 2020 Sep 5;754:144811. doi: 10.1016/j.gene.2020.144811. Epub 2020 May 25. Gene. 2020. PMID: 32464246
-
CircRNA SCARB1 Promotes Renal Cell Carcinoma Progression Via Mir- 510-5p/SDC3 Axis.Curr Cancer Drug Targets. 2020;20(6):461-470. doi: 10.2174/1568009620666200409130032. Curr Cancer Drug Targets. 2020. PMID: 32271695
-
Circular RNAs in renal cell carcinoma: implications for tumorigenesis, diagnosis, and therapy.Mol Cancer. 2020 Oct 14;19(1):149. doi: 10.1186/s12943-020-01266-7. Mol Cancer. 2020. PMID: 33054773 Free PMC article. Review.
-
Circular RNAs in renal cell carcinoma: Functions in tumorigenesis and diagnostic and prognostic potentials.Pathol Res Pract. 2022 Jan;229:153720. doi: 10.1016/j.prp.2021.153720. Epub 2021 Nov 28. Pathol Res Pract. 2022. PMID: 34942510 Review.
Cited by
-
Circular RNAs as Prognostic Biomarkers in Renal Cell Carcinoma: A Systematic Review and Meta-Analysis.Front Genet. 2022 Jun 8;13:878700. doi: 10.3389/fgene.2022.878700. eCollection 2022. Front Genet. 2022. PMID: 35754794 Free PMC article.
-
Synergistic Effects of TW-37 and ABT-263 on Renal Cell Carcinoma Cells.Cancer Manag Res. 2021 Feb 3;13:953-963. doi: 10.2147/CMAR.S265788. eCollection 2021. Cancer Manag Res. 2021. PMID: 33568941 Free PMC article.
-
A multi-modal fusion model with enhanced feature representation for chronic kidney disease progression prediction.Brief Bioinform. 2024 Nov 22;26(1):bbaf003. doi: 10.1093/bib/bbaf003. Brief Bioinform. 2024. PMID: 39913621 Free PMC article.
-
Overexpression of hepatocyte growth factor protects chronic myeloid leukemia cells from apoptosis induced by etoposide.Oncol Lett. 2022 Apr;23(4):122. doi: 10.3892/ol.2022.13242. Epub 2022 Feb 15. Oncol Lett. 2022. PMID: 35261636 Free PMC article.
-
Role of Circular RNA in Kidney-Related Diseases.Front Pharmacol. 2021 Mar 11;12:615882. doi: 10.3389/fphar.2021.615882. eCollection 2021. Front Pharmacol. 2021. PMID: 33776764 Free PMC article.
References
-
- Znaor A, Lortet‐Tieulent J, Laversanne M, Jemal A, Bray F. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol. 2015;67:519‐530. - PubMed
-
- Xing M, Kokabi N, Zhang D, Ludwig JM, Kim HS. Comparative effectiveness of thermal ablation, surgical resection, and active surveillance for T1a renal cell carcinoma: a surveillance, epidemiology, and end results (SEER)‐medicare‐linked population study. Radiology. 2018;288:81‐90. - PubMed
-
- Memczak S, Jens M, Elefsinioti A, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333‐338. - PubMed
-
- Zhang XO, Wang HB, Zhang Y, Lu X, Chen LL, Yang L. Complementary sequence‐mediated exon circularization. Cell. 2014;159:134‐147. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

