Sirt1 sustains female fertility by slowing age-related decline in oocyte quality required for post-fertilization embryo development
- PMID: 32729989
- PMCID: PMC7511857
- DOI: 10.1111/acel.13204
Sirt1 sustains female fertility by slowing age-related decline in oocyte quality required for post-fertilization embryo development
Abstract
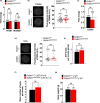
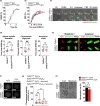
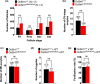
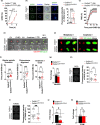
The NAD+ -dependent sirtuin deacetylase, Sirt1, regulates key transcription factors strongly implicated in ageing and lifespan. Due to potential confounding effects secondary to loss of Sirt1 function from the soma in existing whole-animal mutants, the in vivo role of Sirt1 in oocytes (oocyte-Sirt1) for female fertility remains unknown. We deleted Sirt1 specifically in growing oocytes and study how loss of oocyte-Sirt1 affects a comprehensive range of female reproductive parameters including ovarian follicular reservoir, oocyte maturation, oocyte mitochondrial abundance, oxidative stress, fertilization, embryo development and fertility during ageing. Surprisingly, eliminating this key sirtuin from growing oocytes has no effect in young females. During a 10-month-long breeding trial, however, we find that 50% of females lacking oocyte-Sirt1 become prematurely sterile between 9 and 11 months of age when 100% of wild-type females remain fertile. This is not due to an accelerated age-related decline in oocyte numbers in the absence of oocyte-Sirt1 but to reduced oocyte developmental competence or quality. Compromised oocyte quality does not impact in vivo oocyte maturation or fertilization but leads to increased oxidative stress in preimplantation embryos that inhibits cleavage divisions. Our data suggest that defects emerge in aged females lacking oocyte-Sirt1 due to concurrent age-related changes such as reduced NAD+ and sirtuin expression levels, which compromise compensatory mechanisms that can cover for Sirt1 loss in younger oocytes. In contrast to evidence that increasing Sirt1 activity delays ageing, our data provide some of the only in vivo evidence that loss of Sirt1 induces premature ageing.
Keywords: Sirt1; ageing; fertility; mitochondria; oocyte; oxidative stress.
© 2020 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
HAH is a co‐founder, shareholder and advisor of Jumpstart Fertility Inc, which was founded to develop research into NAD+‐dependent pathways involved in female fertility.
Figures

References
-
- Biel, T. G. , Lee, S. , Flores‐Toro, J. A. , Dean, J. W. , Go, K. L. , Lee, M.‐H. , … Kim, J.‐S. (2016). Sirtuin 1 suppresses mitochondrial dysfunction of ischemic mouse livers in a mitofusin 2‐dependent manner. Cell Death and Differentiation, 23(2), 279–290. 10.1038/cdd.2015.96 - DOI - PMC - PubMed
-
- Cheng, H.‐L. , Mostoslavsky, R. , Saito, S. , Manis, J. P. , Gu, Y. , Patel, P. , … Chua, K. F. (2003). Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)‐deficient mice. Proceedings of the National Academy of Sciences of the United States of America, 100(19), 10794–10799. 10.1073/pnas.1934713100 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

