Role of O-Linked N-Acetylglucosamine Protein Modification in Cellular (Patho)Physiology
- PMID: 32730113
- PMCID: PMC8428922
- DOI: 10.1152/physrev.00043.2019
Role of O-Linked N-Acetylglucosamine Protein Modification in Cellular (Patho)Physiology
Abstract
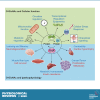
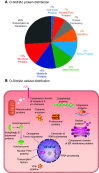
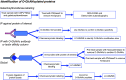
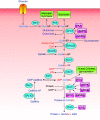
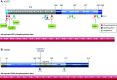
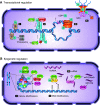
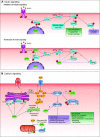
In the mid-1980s, the identification of serine and threonine residues on nuclear and cytoplasmic proteins modified by a N-acetylglucosamine moiety (O-GlcNAc) via an O-linkage overturned the widely held assumption that glycosylation only occurred in the endoplasmic reticulum, Golgi apparatus, and secretory pathways. In contrast to traditional glycosylation, the O-GlcNAc modification does not lead to complex, branched glycan structures and is rapidly cycled on and off proteins by O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA), respectively. Since its discovery, O-GlcNAcylation has been shown to contribute to numerous cellular functions, including signaling, protein localization and stability, transcription, chromatin remodeling, mitochondrial function, and cell survival. Dysregulation in O-GlcNAc cycling has been implicated in the progression of a wide range of diseases, such as diabetes, diabetic complications, cancer, cardiovascular, and neurodegenerative diseases. This review will outline our current understanding of the processes involved in regulating O-GlcNAc turnover, the role of O-GlcNAcylation in regulating cellular physiology, and how dysregulation in O-GlcNAc cycling contributes to pathophysiological processes.
Keywords: calcium; cancer; diabetes; genetics; metabolism.
Figures


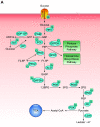

References
-
- Varki A, Kornfeld S. Historical background and overview. In: Essentials of Glycobiology (3rd ed.), edited by Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH, Schnaar RL, Seeberger PH.. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, NY, 2015. - PubMed
-
- Torres CR, Hart GW. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J Biol Chem 259: 3308–3317, 1984. - PubMed
-
- Holt GD, Hart GW. The subcellular distribution of terminal N-acetylglucosamine moieties. Localization of a novel protein-saccharide linkage, O-linked GlcNAc. J Bioll Chem 261: 8049–8057, 1986. - PubMed
-
- Hanover JA, Cohen CK, Willingham MC, Park MK. O-linked N-acetylglucosamine is attached to proteins of the nuclear pore. Evidence for cytoplasmic and nucleoplasmic glycoproteins. J Biol Chem 262: 9887–9894, 1987. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- I01 BX004251/BX/BLRD VA/United States
- R01 HL142216/HL/NHLBI NIH HHS/United States
- R21 HL152354/HL/NHLBI NIH HHS/United States
- R01 HL133011/HL/NHLBI NIH HHS/United States
- HL142216/NH/NIH HHS/United States
- P30 DK079626/DK/NIDDK NIH HHS/United States
- BX004251/VA/VA/United States
- AG050886/NH/NIH HHS/United States
- R56 AG060959/AG/NIA NIH HHS/United States
- R01 AG072895/AG/NIA NIH HHS/United States
- P30 AG050886/AG/NIA NIH HHS/United States
- AG060959/NH/NIH HHS/United States
- P30 DK056336/DK/NIDDK NIH HHS/United States
- HL133011/NH/NIH HHS/United States
- R56 HL133011/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

