Human Rotator Cuff Tears Have an Endogenous, Inducible Stem Cell Source Capable of Improving Muscle Quality and Function After Rotator Cuff Repair
- PMID: 32730704
- PMCID: PMC9262007
- DOI: 10.1177/0363546520935855
Human Rotator Cuff Tears Have an Endogenous, Inducible Stem Cell Source Capable of Improving Muscle Quality and Function After Rotator Cuff Repair
Abstract
Background: The muscle quality of the rotator cuff (RC), measured by atrophy and fatty infiltration (FI), is a key determinant of outcomes in RC injury and repair. The ability to regenerate muscle after repair has been shown to be limited.
Purpose: To determine if there is a source of resident endogenous stem cells, fibroadipogenic progenitor cells (FAPs), within RC injury patients, and if these cells are capable of adipogenic, fibrogenic, and pro-myogenic differentiation.
Study design: Controlled laboratory study.
Methods: A total of 20 patients between the ages of 40 and 75 years with partial- or full-thickness RC tears of the supraspinatus and evidence of atrophy and FI Goutallier grade 1, 2, or 3 were selected from 2 surgeons at an orthopaedic center. During the surgical repair procedure, supraspinatus muscle biopsy specimens were obtained for analysis as were deltoid muscle biopsy specimens to serve as the control. FAPs and satellite cells were quantified using fluorescence-activated cell sorting. Muscle FI and fibrosis was quantified using Oil Red O and Masson trichrome staining. FAP differentiation and gene expression profiles were compared across tear sizes after culture in adipogenic, fibrogenic, and beta-3 agonist (amibegron) conditions. Analysis of variance was used for statistical comparisons between groups, with P < .05 as statistically significant.
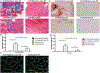
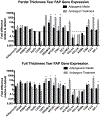
Results: Histologic analysis confirmed the presence of fat in biopsy specimens from patients with full-thickness tears. There were more FAPs in the full-thickness tear group compared with the partial-thickness tear group (9.43% ± 4.25% vs 3.84% ± 2.54%; P < .01). Full-thickness tears were divided by tear size, with patients with larger tears having significantly more FAPs than those with smaller tears. FAPs from muscles with full-thickness tendon tears had more adipogenic and fibrogenic potential than those with partial tears. Induction of a beige adipose tissue (BAT) phenotype in FAPs was possible, as demonstrated by increased expression of BAT markers and pro-myogenic genes including insulin-like growth factor 1 and follistatin.
Conclusion: Endogenous FAPs are present within the RC and likely are the source of FI. These FAPs were increased in muscles with in larger tears but are capable of adopting a pro-myogenic BAT phenotype that could be utilized to improve muscle quality and patient function after RC repair.
Keywords: fibroadipogenic progenitor; rotator cuff; shoulder; stem cell.
Figures


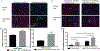
References
-
- Collins CA, Zammit PS, Ruiz AP, et al. A population of myogenic stemcells that survives skeletal muscle aging. Stem Cells. 2007;25(4):885–894. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

