ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques
- PMID: 32731258
- PMCID: PMC8436420
- DOI: 10.1038/s41586-020-2608-y
ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques
Erratum in
-
Publisher Correction: ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques.Nature. 2021 Feb;590(7844):E24. doi: 10.1038/s41586-020-03099-2. Nature. 2021. PMID: 33469217 No abstract available.
Abstract
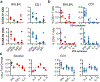
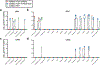
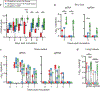
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in December 20191,2 and is responsible for the coronavirus disease 2019 (COVID-19) pandemic3. Vaccines are an essential countermeasure and are urgently needed to control the pandemic4. Here we show that the adenovirus-vector-based vaccine ChAdOx1 nCoV-19, which encodes the spike protein of SARS-CoV-2, is immunogenic in mice and elicites a robust humoral and cell-mediated response. This response was predominantly mediated by type-1 T helper cells, as demonstrated by the profiling of the IgG subclass and the expression of cytokines. Vaccination with ChAdOx1 nCoV-19 (using either a prime-only or a prime-boost regimen) induced a balanced humoral and cellular immune response of type-1 and type-2 T helper cells in rhesus macaques. We observed a significantly reduced viral load in the bronchoalveolar lavage fluid and lower respiratory tract tissue of vaccinated rhesus macaques that were challenged with SARS-CoV-2 compared with control animals, and no pneumonia was observed in vaccinated SARS-CoV-2-infected animals. However, there was no difference in nasal shedding between vaccinated and control SARS-CoV-2-infected macaques. Notably, we found no evidence of immune-enhanced disease after viral challenge in vaccinated SARS-CoV-2-infected animals. The safety, immunogenicity and efficacy profiles of ChAdOx1 nCoV-19 against symptomatic PCR-positive COVID-19 disease will now be assessed in randomized controlled clinical trials in humans.
Conflict of interest statement
Competing interests
SCG is a board member of Vaccitech and named as an inventor on a patent covering use of ChAdOx1-vectored vaccines and a patent application covering a SARS-CoV-2 (nCoV-19) vaccine (UK Patent Application No. 2003670.3). Teresa Lambe is named as an inventor on a patent application covering a SARS-CoV-2 (nCoV-19) vaccine (UK Patent Application No. 2003670.3). The University of Oxford and Vaccitech, having joint rights in the vaccine, entered into a partnership with AstraZeneca in April 2020 for further development, large-scale manufacture and global supply. Equitable access to the vaccine is a key component of the partnership. Neither Oxford University nor Vaccitech will receive any royalties during the pandemic period or from any sales of the vaccine in developing countries. The remaining authors declare no competing interests.
Figures







Update of
-
ChAdOx1 nCoV-19 vaccination prevents SARS-CoV-2 pneumonia in rhesus macaques.bioRxiv [Preprint]. 2020 May 13:2020.05.13.093195. doi: 10.1101/2020.05.13.093195. bioRxiv. 2020. Update in: Nature. 2020 Oct;586(7830):578-582. doi: 10.1038/s41586-020-2608-y. PMID: 32511340 Free PMC article. Updated. Preprint.
References
-
- WHO. Coronavirus disease (COVID-19) Situation Report 113, <https://www.who.int/docs/default-source/coronaviruse/situation-reports/2...> (2020).
References Methods
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

