A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenström macroglobulinemia: the ASPEN study
- PMID: 32731259
- PMCID: PMC7596850
- DOI: 10.1182/blood.2020006844
A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenström macroglobulinemia: the ASPEN study
Abstract
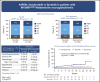
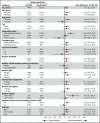
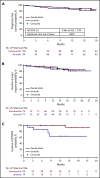
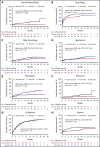
Bruton tyrosine kinase (BTK) inhibition is an effective treatment approach for patients with Waldenström macroglobulinemia (WM). The phase 3 ASPEN study compared the efficacy and safety of ibrutinib, a first-generation BTK inhibitor, with zanubrutinib, a novel highly selective BTK inhibitor, in patients with WM. Patients with MYD88L265P disease were randomly assigned 1:1 to treatment with ibrutinib or zanubrutinib. The primary end point was the proportion of patients achieving a complete response (CR) or a very good partial response (VGPR) by independent review. Key secondary end points included major response rate (MRR), progression-free survival (PFS), duration of response (DOR), disease burden, and safety. A total of 201 patients were randomized, and 199 received ≥1 dose of study treatment. No patient achieved a CR. Twenty-nine (28%) zanubrutinib patients and 19 (19%) ibrutinib patients achieved a VGPR, a nonstatistically significant difference (P = .09). MRRs were 77% and 78%, respectively. Median DOR and PFS were not reached; 84% and 85% of ibrutinib and zanubrutinib patients were progression free at 18 months. Atrial fibrillation, contusion, diarrhea, peripheral edema, hemorrhage, muscle spasms, and pneumonia, as well as adverse events leading to treatment discontinuation, were less common among zanubrutinib recipients. Incidence of neutropenia was higher with zanubrutinib, although grade ≥3 infection rates were similar in both arms (1.2 and 1.1 events per 100 person-months). These results demonstrate that zanubrutinib and ibrutinib are highly effective in the treatment of WM, but zanubrutinib treatment was associated with a trend toward better response quality and less toxicity, particularly cardiovascular toxicity.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: C.S.T. receives research funding from Janssen and AbbVie and receives honoraria from Janssen, AbbVie, BeiGene, Novartis, and Roche. S.O. consults for AbbVie, Janssen, Gilead Sciences, Roche, Mundipharma, Merck, Bristol Myers Squibb, and Celgene; receives research funding from AbbVie, BeiGene, Janssen, Gilead Sciences, Roche, Celgene, and Epizyme; and receives honoraria from AbbVie, Janssen, Gilead Sciences, Roche, Mundipharma, Merck, Bristol Myers Squibb, and Celgene. S.D. participates in the speaker’s bureau for Amgen, receives research funding from Janssen and BeiGene, and has travel expenses paid by Janssen. W.J. consults for AstraZeneca, Debiopharm, Janssen, Gilead Sciences, and Roche and receives research funding from Acerta, AstraZeneca, Janssen, BeiGene, Bayer, Celltrion, Debiopharm, Epizyme, Merck, MorphoSys, MEI Pharma, Servier, Roche, and TG Therapeutics. H.-P.L. has equity ownership in CSL Behring, receives honoraria from Roche, and has travel expenses paid by AbbVie. G.C. receives research funding from BeiGene, Acerta, and Glycomimetics. R.G.O. receives honoraria from Janssen and Celgene and consults for, and has travel expenses paid by, Janssen. B.E.W. consults for Roche and receives research funding from Roche and Gilead Sciences. R.G.S. consults for Janssen; receives honoraria from Janssen, Takeda, and Amgen; receives research funding from Gilead Sciences and Incyte; and has travel expenses paid by Janssen and Takeda. H.M. receives honoraria from Janssen and consults for AstraZeneca. A.T. consults for and receives honoraria from Janssen SpA, AstraZeneca, and AbbVie. J.C. consults for BeiGene, Janssen, Kymera Therapeutics, and Pharmacyclics and receives research funding from AbbVie, BeiGene, Janssen, Pharmacyclics, and TG Therapeutics. C.F.d.L. consults for and receives honoraria from Janssen, Celgene, and Amgen and receives research funding from, and has travel expenses paid by, Janssen, Celgene, Amgen, and Takeda. D.B. consults for, receives research funding from, and has travel expenses paid by Roche, Takeda, and Gilead Sciences. E.L. consults for Akcea Therapeutics, Adaptive Phage Therapeutics, and Pharmacyclics. J.M. consults for Celgene and Pharmacyclics, receives honoraria from Celgene, and participates in the speaker’s bureau for Celgene. M.M. consults for Roche and Janssen. T.S. consults for AstraZeneca, Kite Pharma, Juno Therapeutics, and BeiGene; receives research funding from Pharmacyclics, Juno, BeiGene, Astra Zeneca, TG Therapeutics, and Celgene; and participates in the speaker’s bureau for Pharmacyclics, Janssen, AstraZeneca, and Seattle Genetics. M. Trneny is an employee of Charles University General Hospital; receives honoraria from Janssen, Gilead Sciences, Takeda, Bristol Myers Squibb, Amgen, AbbVie, Roche, MorphoSys, and Incyte; and consults for Takeda, Bristol Myers Squibb, Incyte, AbbVie, Amgen, Roche, Gilead Sciences, Janssen, Celgene, and MorphoSys. M.C.M. consults for Kite/Gilead and Servier and has travel expenses paid by Celgene. C.B. receives honoraria from, consults for, and participates in the speaker’s bureau for Roche, Janssen, Celltrion, and BeiGene and receives research funding from Roche, Janssen, and BeiGene. V.L. receives honoraria from AstraZeneca, Roche, Gilead Sciences, Amgen, AbbVie, and Janssen; consults for AstraZeneca, AbbVie, Roche, and Janssen; participates in the speaker’s bureau for AbbVie and Janssen; and has travel expenses paid by AbbVie, Roche, and Janssen. J.T. receives research funding from BeiGene, Celgene, a Bristol-Myers Squibb Company, Pharmacyclics, Roche, and Takeda. W.Y.C. is an employee of BeiGene and has equity ownership in BeiGene and Bristol Myers Squibb. J.S. is an employee of and has equity ownership in BeiGene. S.R. is an employee of BeiGene, has equity ownership in BeiGene and Amgen, and holds patents and receives royalties from Roche Molecular Diagnostics. A.C. is an employee of, has equity ownership in, and has travel expenses paid by BeiGene. J.H. is an employee of, has a leadership role at, and has equity ownership in BeiGene. M.D. consults for and receives honoraria from Amgen, Janssen, Takeda, Celgene, and Bristol Myers Squibb. The remaining authors declare no competing financial interests.
Figures
References
-
- Gertz MA. Waldenström macroglobulinemia: 2019 update on diagnosis, risk stratification, and management. Am J Hematol. 2019;94(2):266-276. - PubMed
-
- Treon SP, Xu L, Yang G, et al. MYD88 L265P somatic mutation in Waldenström’s macroglobulinemia. N Engl J Med. 2012;367(9):826-833. - PubMed
-
- Cao Y, Hunter ZR, Liu X, et al. CXCR4 WHIM-like frameshift and nonsense mutations promote ibrutinib resistance but do not supplant MYD88(L265P) -directed survival signalling in Waldenström macroglobulinaemia cells. Br J Haematol. 2015;168(5):701-707. - PubMed
-
- Cao Y, Hunter ZR, Liu X, et al. The WHIM-like CXCR4(S338X) somatic mutation activates AKT and ERK, and promotes resistance to ibrutinib and other agents used in the treatment of Waldenstrom’s macroglobulinemia. Leukemia. 2015;29(1):169-176. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

