Chiral Amphiphilic Secondary Amine-Porphyrin Hybrids for Aqueous Organocatalysis
- PMID: 32731520
- PMCID: PMC7435841
- DOI: 10.3390/molecules25153420
Chiral Amphiphilic Secondary Amine-Porphyrin Hybrids for Aqueous Organocatalysis
Abstract
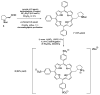
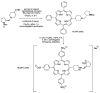
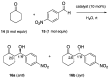
Two chiral proline-derived amphiphilic 5-substituted-10,15,20-tris(4-sulfonatophenyl)porphyrins were prepared, and their pH-dependent supramolecular behavior was studied. In neutral aqueous solutions, the free-base form of the hybrids is highly soluble, allowing enamine-based organocatalysis to take place, whereas under acidic conditions, the porphyrinic protonated core of the hybrid leads to the formation of self-assembled structures, so that the hybrids flocculate and their catalytic activity is fully suppressed. The low degree of chirality transfer observed for aqueous Michael and aldol reactions strongly suggests that these reactions take place under true "in water" organocatalytic conditions. The highly insoluble catalyst aggregates can easily be separated from the reaction products by centrifugation of the acidic reaction mixtures, and after neutralization and desalting, the sodium salts of the sulfonated amine-porphyrin hybrids, retaining their full catalytic activity, can be recovered in high yield.
Keywords: J-aggregates; Michael reaction; aldol reaction; aqueous organocatalysis; porphyrins; switchable catalysis.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


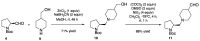



Similar articles
-
Synthesis of New Amino-Functionalized Porphyrins:Preliminary Study of Their Organophotocatalytic Activity.Molecules. 2023 Feb 20;28(4):1997. doi: 10.3390/molecules28041997. Molecules. 2023. PMID: 36838985 Free PMC article.
-
Chiral Recognition of L- and D- Amino Acid by Porphyrin Supramolecular Aggregates.Molecules. 2018 Dec 27;24(1):84. doi: 10.3390/molecules24010084. Molecules. 2018. PMID: 30591641 Free PMC article.
-
Tunable Supramolecular Chirogenesis in the Self-Assembling of Amphiphilic Porphyrin Triggered by Chiral Amines.Int J Mol Sci. 2020 Nov 13;21(22):8557. doi: 10.3390/ijms21228557. Int J Mol Sci. 2020. PMID: 33202819 Free PMC article.
-
New concepts for organocatalysis.Ernst Schering Found Symp Proc. 2007;(2):1-43. Ernst Schering Found Symp Proc. 2007. PMID: 18646300 Review.
-
The Self-Aggregation of Porphyrins with Multiple Chiral Centers in Organic/Aqueous Media: The Case of Sugar- and Steroid-Porphyrin Conjugates.Molecules. 2020 Oct 4;25(19):4544. doi: 10.3390/molecules25194544. Molecules. 2020. PMID: 33020381 Free PMC article. Review.
Cited by
-
Photocatalytic Water Splitting Promoted by 2D and 3D Porphyrin Covalent Organic Polymers Synthesized by Suzuki-Miyaura Carbon-Carbon Coupling.Nanomaterials (Basel). 2022 Sep 14;12(18):3197. doi: 10.3390/nano12183197. Nanomaterials (Basel). 2022. PMID: 36144987 Free PMC article.
-
Switchable aqueous catalytic systems for organic transformations.Commun Chem. 2022 Sep 26;5(1):115. doi: 10.1038/s42004-022-00734-z. Commun Chem. 2022. PMID: 36697818 Free PMC article. Review.
-
Synthesis of New Amino-Functionalized Porphyrins:Preliminary Study of Their Organophotocatalytic Activity.Molecules. 2023 Feb 20;28(4):1997. doi: 10.3390/molecules28041997. Molecules. 2023. PMID: 36838985 Free PMC article.
-
Substituent Effects in the Photophysical and Electrochemical Properties of Meso-Tetraphenylporphyrin Derivatives.Molecules. 2024 Aug 4;29(15):3689. doi: 10.3390/molecules29153689. Molecules. 2024. PMID: 39125093 Free PMC article.
-
Design and Synthesis of Helical N-Terminal L-Prolyl Oligopeptides Possessing Hydrocarbon Stapling.Molecules. 2020 Oct 13;25(20):4667. doi: 10.3390/molecules25204667. Molecules. 2020. PMID: 33066194 Free PMC article.

