Rapid Fabrication of Membrane-Integrated Thermoplastic Elastomer Microfluidic Devices
- PMID: 32731570
- PMCID: PMC7463978
- DOI: 10.3390/mi11080731
Rapid Fabrication of Membrane-Integrated Thermoplastic Elastomer Microfluidic Devices
Abstract
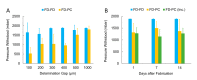
Leveraging the advantageous material properties of recently developed soft thermoplastic elastomer materials, this work presents the facile and rapid fabrication of composite membrane-integrated microfluidic devices consisting of FlexdymTM polymer and commercially available porous polycarbonate membranes. The three-layer devices can be fabricated in under 2.5 h, consisting of a 2-min hot embossing cycle, conformal contact between device layers and a low-temperature baking step. The strength of the FlexdymTM-polycarbonate seal was characterized using a specialized microfluidic delamination device and an automated pressure controller configuration, offering a standardized and high-throughput method of microfluidic burst testing. Given a minimum bonding distance of 200 μm, the materials showed bonding that reliably withstood pressures of 500 mbar and above, which is sufficient for most microfluidic cell culture applications. Bonding was also stable when subjected to long term pressurization (10 h) and repeated use (10,000 pressure cycles). Cell culture trials confirmed good cell adhesion and sustained culture of human dermal fibroblasts on a polycarbonate membrane inside the device channels over the course of one week. In comparison to existing porous membrane-based microfluidic platforms of this configuration, most often made of polydimethylsiloxane (PDMS), these devices offer a streamlined fabrication methodology with materials having favourable properties for cell culture applications and the potential for implementation in barrier model organ-on-chips.
Keywords: delamination testing; membrane-based cell culture; microfluidic device; rapid fabrication; thermoplastic elastomer.
Conflict of interest statement
The authors A.H.M., E.K.T., A.D. and S.C.L.P., at the time of this work, were employees of Elvesys SAS, a for profit company that sells Elveflow® equipment, which was used for flow control and measurements in this work.
Figures




Similar articles
-
Facile Patterning of Thermoplastic Elastomers and Robust Bonding to Glass and Thermoplastics for Microfluidic Cell Culture and Organ-on-Chip.Micromachines (Basel). 2021 May 18;12(5):575. doi: 10.3390/mi12050575. Micromachines (Basel). 2021. PMID: 34070209 Free PMC article.
-
Microfluidic device fabrication by thermoplastic hot-embossing.Methods Mol Biol. 2013;949:115-23. doi: 10.1007/978-1-62703-134-9_8. Methods Mol Biol. 2013. PMID: 23329439
-
Microfluidic device fabrication mediated by surface chemical bonding.Analyst. 2020 Jun 15;145(12):4096-4110. doi: 10.1039/d0an00614a. Analyst. 2020. PMID: 32451519
-
Polymer Microfluidics: Simple, Low-Cost Fabrication Process Bridging Academic Lab Research to Commercialized Production.Micromachines (Basel). 2016 Dec 10;7(12):225. doi: 10.3390/mi7120225. Micromachines (Basel). 2016. PMID: 30404397 Free PMC article. Review.
-
Recent Advances in Thermoplastic Microfluidic Bonding.Micromachines (Basel). 2022 Mar 20;13(3):486. doi: 10.3390/mi13030486. Micromachines (Basel). 2022. PMID: 35334777 Free PMC article. Review.
Cited by
-
Lung on a Chip Development from Off-Stoichiometry Thiol-Ene Polymer.Micromachines (Basel). 2021 May 11;12(5):546. doi: 10.3390/mi12050546. Micromachines (Basel). 2021. PMID: 34064627 Free PMC article.
-
Reducing Inert Materials for Optimal Cell-Cell and Cell-Matrix Interactions within Microphysiological Systems.Biomimetics (Basel). 2024 Apr 25;9(5):262. doi: 10.3390/biomimetics9050262. Biomimetics (Basel). 2024. PMID: 38786472 Free PMC article.
-
A flexible strategy to fabricate trumpet-shaped porous PDMS membranes for organ-on-chip application.Biomicrofluidics. 2024 Sep 5;18(5):054101. doi: 10.1063/5.0227148. eCollection 2024 Sep. Biomicrofluidics. 2024. PMID: 39247799
-
Comparison of Printable Biomaterials for Use in Neural Tissue Engineering: An In Vitro Characterization and In Vivo Biocompatibility Assessment.Polymers (Basel). 2024 May 17;16(10):1426. doi: 10.3390/polym16101426. Polymers (Basel). 2024. PMID: 38794619 Free PMC article.
-
Low-Cost, Open-Source, High-Precision Pressure Controller for Multi-Channel Microfluidics.Biosensors (Basel). 2025 Mar 2;15(3):154. doi: 10.3390/bios15030154. Biosensors (Basel). 2025. PMID: 40136951 Free PMC article.
References
-
- Lin H., Li H., Cho H.-J., Bian S., Roh H.-J., Lee M.-K., Kim J.S., Chung S.-J., Shim C.-K., Kim D.-D. Air-Liquid Interface (ALI) Culture of Human Bronchial Epithelial Cell Monolayers as an in vitro Model for Airway Drug Transport Studies. J. Pharm. Sci. 2007;96:341–350. doi: 10.1002/jps.20803. - DOI - PubMed
-
- Harisi R., Kenessey I., Olah J.N., Timar F., Babo I., Pogany G., Paku S., Jeney A. Differential inhibition of single and cluster type tumor cell migration. Anticancer Res. 2009;29:2981–2985. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

