DNA Damage-Inducing Anticancer Therapies: From Global to Precision Damage
- PMID: 32731592
- PMCID: PMC7463878
- DOI: 10.3390/cancers12082098
DNA Damage-Inducing Anticancer Therapies: From Global to Precision Damage
Abstract
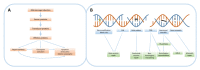
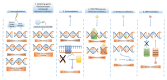
DNA damage-inducing therapies are of tremendous value for cancer treatment and function by the direct or indirect formation of DNA lesions and subsequent inhibition of cellular proliferation. Of central importance in the cellular response to therapy-induced DNA damage is the DNA damage response (DDR), a protein network guiding both DNA damage repair and the induction of cancer-eradicating mechanisms such as apoptosis. A detailed understanding of DNA damage induction and the DDR has greatly improved our knowledge of the classical DNA damage-inducing therapies, radiotherapy and cytotoxic chemotherapy, and has paved the way for rational improvement of these treatments. Moreover, compounds targeting specific DDR proteins, selectively impairing DNA damage repair in cancer cells, form a promising novel therapy class that is now entering the clinic. In this review, we give an overview of the current state and ongoing developments, and discuss potential avenues for improvement for DNA damage-inducing therapies, with a central focus on the role of the DDR in therapy response, toxicity and resistance. Furthermore, we describe the relevance of using combination regimens containing DNA damage-inducing therapies and how they can be utilized to potentiate other anticancer strategies such as immunotherapy.
Keywords: DDR modulators; DNA damage response; DNA damage-inducing therapies; DNA repair; cancer therapy; combination therapies; cytotoxic chemotherapy; radiotherapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

