Single Residue Substitution at N-Terminal Affects Temperature Stability and Activity of L2 Lipase
- PMID: 32731608
- PMCID: PMC7435863
- DOI: 10.3390/molecules25153433
Single Residue Substitution at N-Terminal Affects Temperature Stability and Activity of L2 Lipase
Abstract
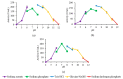
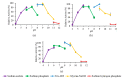
Rational design is widely employed in protein engineering to tailor wild-type enzymes for industrial applications. The typical target region for mutation is a functional region like the catalytic site to improve stability and activity. However, few have explored the role of other regions which, in principle, have no evident functionality such as the N-terminal region. In this study, stability prediction software was used to identify the critical point in the non-functional N-terminal region of L2 lipase and the effects of the substitution towards temperature stability and activity were determined. The results showed 3 mutant lipases: A8V, A8P and A8E with 29% better thermostability, 4 h increase in half-life and 6.6 °C higher thermal denaturation point, respectively. A8V showed 1.6-fold enhancement in activity compared to wild-type. To conclude, the improvement in temperature stability upon substitution showed that the N-terminal region plays a role in temperature stability and activity of L2 lipase.
Keywords: homology modelling; lipase; rational design; stability prediction; thermostability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
The Effects of One Amino Acid Substitutions at the C-Terminal Region of Thermostable L2 Lipase by Computational and Experimental Approach.Mol Biotechnol. 2018 Jan;60(1):1-11. doi: 10.1007/s12033-017-0038-3. Mol Biotechnol. 2018. PMID: 29058211
-
Engineering lipases for temperature adaptation: Structure function correlation.Biochim Biophys Acta Proteins Proteom. 2019 Nov;1867(11):140261. doi: 10.1016/j.bbapap.2019.08.001. Epub 2019 Aug 8. Biochim Biophys Acta Proteins Proteom. 2019. PMID: 31401312 Review.
-
Enhancing the Thermostability of Rhizomucor miehei Lipase with a Limited Screening Library by Rational-Design Point Mutations and Disulfide Bonds.Appl Environ Microbiol. 2018 Jan 2;84(2):e02129-17. doi: 10.1128/AEM.02129-17. Print 2018 Jan 15. Appl Environ Microbiol. 2018. PMID: 29101200 Free PMC article.
-
Directed Evolution of Recombinant C-Terminal Truncated Staphylococcus epidermidis Lipase AT2 for the Enhancement of Thermostability.Int J Mol Sci. 2017 Nov 4;18(11):2202. doi: 10.3390/ijms18112202. Int J Mol Sci. 2017. PMID: 29113034 Free PMC article.
-
Improving the Catalytic Activity and Thermostability of MAS1 Lipase by Alanine Substitution.Mol Biotechnol. 2018 Apr;60(4):319-328. doi: 10.1007/s12033-018-0062-y. Mol Biotechnol. 2018. PMID: 29450814 Review.
Cited by
-
Crucial Residues of C-Terminal Oligopeptide C60 to Improve the Yield of Prebiotic Xylooligosaccharides by Truncated Mutation.Foods. 2022 Mar 18;11(6):862. doi: 10.3390/foods11060862. Foods. 2022. PMID: 35327284 Free PMC article.
-
Enhancement of enzymatic activity by biomolecular condensates through pH buffering.Nat Commun. 2025 Jul 10;16(1):6368. doi: 10.1038/s41467-025-61013-8. Nat Commun. 2025. PMID: 40640131 Free PMC article.
-
In Silico Analysis and Development of the Secretory Expression of D-Psicose-3-Epimerase in Escherichia coli.Microorganisms. 2024 Aug 1;12(8):1574. doi: 10.3390/microorganisms12081574. Microorganisms. 2024. PMID: 39203416 Free PMC article.
-
Discovery of the Key Mutation Site Influencing the Thermostability of Thermomyces lanuginosus Lipase by Rosetta Design Programs.Int J Mol Sci. 2022 Aug 11;23(16):8963. doi: 10.3390/ijms23168963. Int J Mol Sci. 2022. PMID: 36012226 Free PMC article.
References
-
- Sangeetha R., Arulpandi I., Geetha A. Bacterial Lipases as Potential Industrial Biocatalysts: An Overview. Res. J. Microbiol. 2011;6:1–24. doi: 10.3923/jm.2011.1.24. - DOI
-
- Kapoor M., Gupta M.N. Lipase promiscuity and its biochemical applications. Process. Biochem. 2012;47:555–569. doi: 10.1016/j.procbio.2012.01.011. - DOI
-
- Verma N., Thakur S., Bhatt A.K. Microbial lipases: Industrial applications and properties (a review) Int. Res. J. Biol. Sci. 2012;1:88–92.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

